Coating electrodes with an aerophobic gel significantly enhances the efficiency of gas evolution reactions by preventing bubble coalescence. These bubble-repellant electrodes could find applications in electrochemical devices like water electrolysers and fuel cells.
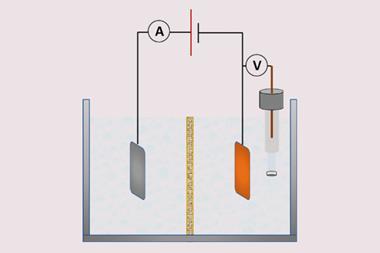
During reactions that form gases, bubbles sticking to electrode surfaces can obstruct catalytic active sites, hinder mass transport and exert mechanical stress on electrodes. Existing ways of modifying electrodes to prevent this, such as electrodeposition or micropatterning, tend to be time, energy and cost-intensive.
Now, researchers from South Korea have developed an approach to prevent sticking bubbles by spin-coating platinum electrodes with a thin layer of polyallylamine hydrochloride (PAH)-based hydrogels. The polyallylamine is crosslinked via Schiff-base condensation reactions that form a coat of 3D fibrous networks roughly 10 μm thick. The process takes less than five minutes at room temperature and uses commercially available materials.
The extremely hydrophilic and aerophobic coating physically separates bubble adhesion and catalytically active sites and decreases the duration of bubble adsorption. Despite a slight reduction in electrochemically active surface area, PAH-modified nickel and platinum electrodes showed improved performance in hydrogen evolution, hydrazine oxidation, and oxygen evolution reactions compared to pristine electrodes. The aerophobic coatings also help to prevent bubble formation from damaging the catalyst surface, prolonging the electrode’s lifespan.
References
Y Kang et al, Adv. Funct. Mater., 2023, DOI: 10.1002/adfm.202308827













No comments yet