Palladium catalyst exploits usually hard-to-access configuration to achieve C–H activation
You might think the US-based organic chemists who have forced their molecules into a boat were attempting some sort of scientific kidnap – but you’d be wrong. Twisting alicyclic amine ring structures into hard-to-access boat-shaped configurations carried Melanie Sanford’s team at the University of Michigan in Ann Arbor to forming carbon–carbon bonds at extremely unreactive sites.
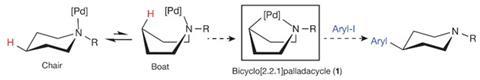
To their amine rings’ nitrogen atoms, the researchers attach both a palladium catalyst and a coordinating group that helps the catalyst flex towards the opposite side of the ring. There, the palladium atom inserts itself into an otherwise difficult to modify carbon–hydrogen bond, enabling the scientists to displace the hydrogen with aromatic rings. ‘We anticipate that this will be an enabling reaction, particularly for medicinal chemists to rapidly access new chemical matter during drug discovery and development,’ Sanford tells Chemistry World.
The idea to twist rings into boats with palladium catalysts sat on the back like an outboard motor came from team member Joseph Topczewski, now at the University of Minnesota. But it wasn’t obvious it would be successful, Sanford observes, and not just because boat-shaped rings tend to flop back to their more common chair-shaped arrangement. ‘The amine functional groups are known to be prone to side reactions in the presence of metals,’ she says. ‘Therefore, we needed to figure out a way to harness the amine in the right way in order to avoid these side reactions.’
The search for suitable conditions alighted on a fluoroamide co-ordinating group that holds the palladium in place in boat shape, and a caesium salt to avoid amine side reactions. The method is ‘pretty operationally simple’ Sanford says, with removing the co-ordinating group ‘probably the hardest part’ because it currently requires tricky-to-use and fairly expensive samarium iodide.
Finding an alternative removal approach is therefore one of the team’s first priorities. However, Sanford is adamant that some chemists will nevertheless find the reaction useful. ‘In medicinal chemistry people work on small scale and the main objective is to get a large number of different derivatives of a core structure, rather than achieve high yields with inexpensive reagents,’ she stresses.
Matthew Gaunt, who studies similar carbon–hydrogen bond activation techniques at the University of Cambridge, UK, calls the approach ‘elegant’. ‘C–H activation of aliphatic amines continues to be a major challenge for synthetic chemists and this exciting paper will clearly advance our capabilities in this important field,’ he adds.
References
J J Topcewski et al, Nature, 2016, DOI: 10.1038/nature16957











No comments yet