Researchers in the US have looked to biology to develop an anionic organic photocatalyst that can reduce arenes with visible light at room temperature. This super-reducing catalyst makes such reactions safer, cuts reaction times and could be expanded to other hard-to-reduce compounds.
Arenes, or aromatic hydrocarbons, are useful starting materials for many commodity chemicals. The Birch reduction is the usual port of call for chemists wanting to reduce arenes, such as benzene, into 1,4-cyclodiene derivatives. Here, pyrophoric alkali metals and a proton source are dissolved in a toxic ammonia solution to generate the solvated electrons needed to drive the reduction. Such harsh conditions often limit the scaling up of reactions. Other methods developed to circumvent the Birch reduction can require complicated set-ups, expensive reagents or need cryogenic or oxygen-sensitive materials.
Now, Niels Damrauer and Garret Miyake, at The Centre for Sustainable Photoredox Catalysis, have co-developed an organic photoredox catalyst that can help avoid standard arene reduction conditions. Photoredox catalysts work by harnessing the energy of light to promote the movement of electrons between species. Metal-based complexes have long-lived excited states, while their organic counterparts are more prone to back electron transfer between species, reforming the original ground state molecules and limiting product formation. ‘We’re trying to avoid the use of catalysts that involve transition metals or very rare elements, [yet] organic photocatalysts… [often] don’t have enough [energy] to do this kind of reduction,’ Damrauer says.
The team took inspiration from chlorophyll P680, a biological electron donor system involved in the reduction of tyrosine. A nearby histidine base within the enzyme abstracts a proton, facilitating proton-coupled electron transfer that reduces back electron transfer.

Through an iterative process of catalyst development, the scientists found that the best candidate was a benzo[α]coronene diester. Mechanistic studies later revealed that the 2e-/1H+ reduced form was responsible for catalysis. ‘By adding two electrons and one proton, [this] is equivalent to a hydride,’ Damrauer adds. This species has a highly reducing excited state and can suppress back electron transfer through proton abstraction from the catalyst itself.
A coupled reaction between the anionic catalyst and the substrate occurs in the presence of a base and visible light, in turn reducing the arene. The unique catalyst action allows for the use of visible light, which avoids undesirable side reactions. ‘With just light and a catalyst, it is quite exciting that you can access this kind of highly reducing condition,’ comments Jean-François Soulé , an expert in light-driven catalysis from ChimieParisTech in France.
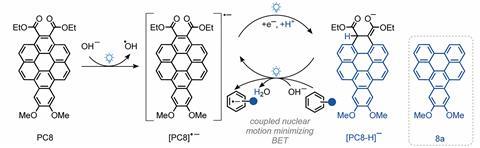
‘No other catalysts have the ability to drive these transformations so efficiently’, Miyake tells Chemistry World. Yields ranged from 23–93% across a diverse range of compounds, with reactions completing in a few hours. A mixture of water, methanol and THF alleviates the need for the harsh solvents used in the standard Birch reduction.
Future work could see these catalysts used on other hard to reduce molecules, such as per- and polyfluoroalkyl substances, or heteroarenes.













No comments yet