
Any reaction that takes place in solution requires stirring. From undergraduate experiments to high-end cutting-edge syntheses, stirring ensures that reagents mix and react under controlled conditions. Magnetic stirrers – ubiquitous spinning workhorses – are at the centre of this process. Found in millions of labs around the world, they are almost universally trusted to do their job without question.
But what if this trust is misplaced? Previously, our research revealed that many of these stirrer bars are contaminated with minute amounts of catalytic metals that can alter experiments and lead to ‘phantom’ catalyst-free reactions.
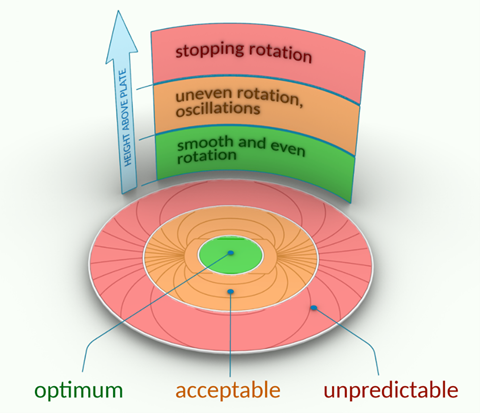
Now, we are reporting a new hidden and surprisingly potent source of experimental variability: the position of a reaction vessel on a magnetic stirrer can significantly affect how well a reaction proceeds.1 Depending on where a vial is placed – even just a few centimetres apart on the same stir plate – reaction rates, yields, nanoparticle size and even product composition can shift unexpectedly. In some cases, the bar may not reach certain ‘dead’ zones in the reaction vessel or even stop spinning entirely.
The signs of stirring problems
Stirrer plates vary in their magnetic field strength and distribution. In many devices, the strength drops off dramatically as you move away from the centre. This affects how the magnetic bar moves – changing from smooth spinning in the centre, to chaotic vibrations at the edges, to complete stalling in elevated or off-centre setups, such as oil baths or multivessel stands. We’ve documented these effects in real-time using time-lapse photography, NMR and electron microscopy.
In parallel reactions, using the same conditions to synthesise nanoparticles, we observed more than two-fold differences in nanoparticle size that were the result of misaligned stirrer bars. In Suzuki-Miyaura cross-coupling reactions, conversions varied by over 10–20% among identical vials placed on the same stirrer. Physical damage to vials – from oscillating bars grinding against the glass walls – has even been detected.
A sensitive issue
A natural assumption is that such effects matter only for heterogeneous systems – where the catalyst is in a different phase to the reactants – that involve solids, catalysts on supports or immiscible phases. Indeed, these are the most obviously sensitive to stirring variations. However, even seemingly homogeneous systems, where the catalyst and reactant are in the same phase, are not immune.
Several critical aspects connect our findings to homogeneous catalysis:
1. Latent heterogeneity – Many homogeneous reactions generate nanoparticles during catalysis, creating what’s known as ‘cocktail-type’ catalysis. These phases behave like heterogeneous systems.
2. Colloidal states – Transient colloids often form during solution reactions and respond strongly to local stirring conditions.
3. Temperature and viscosity gradients – Even in a uniform liquid, stirring is vital to distribute heat and manage viscosity differences that can influence selectivity and yield.
4. Reagent addition dynamics – Gradual addition of reagents, such as in titrations or multi-step dosing, creates transient concentration gradients that only effective stirring can homogenise.
5. Photochemical systems – In these, the light only penetrates part of the solution, creating local reaction zones. Without proper mixing, photobleaching or inconsistent irradiation can affect reproducibility.
While some homogeneous reactions may appear less sensitive to stirring at first glance, nothing can be taken for granted. Many systems, especially those involving fine mechanistic balance, are surprisingly dependent on the integrity of the stirring process.
Can you spot the problem in your lab?
Start with simple visual checks. Run two identical reactions in vials placed at different positions on your magnetic stirrer plate. If you see differences in colour change, yield or product profile, take a closer look. Look how the stirring bar moves inside the reaction vessel – chaotic or oscillating bar motion is a sign of trouble. If your bar rattles, stalls or drifts during a reaction, then stirring is compromised. Similarly, if a nanoparticle reaction gives variable deposit sizes depending on vial location, the culprit may be poor mixing.

You can test stir bar behaviour by filming through the side of a vial or using a magnetic material (nickel or iron granules) spread across the stir plate to map magnetic field lines.
Simple fixes
The good news is: if the issue is detectable, it can be fixed.
My most important advice is:
- Perform a control experiment – especially during final data collection. If you’re working with elevated setups (oil baths, multi-position stands), validate the position first.
- Always run a control experiment with a single reaction vessel placed in the stirrer’s central ‘green zone’ – where bar motion is smooth and uninterrupted. This will help you assess how well parallel reactions represent the true behaviour of your system.
- Document stirrer model, bar type and size, stirring speed and vessel geometry in your experimental section – this level of detail is rare in publications, but necessary for true reproducibility. We are also urging manufacturers to publish stirrer zone maps or provide visualisation aids to help users identify optimal placement areas.
A stirring reminder for better chemistry
Reproducibility doesn’t just depend on pure reagents, new modern equipment and robust protocols. It also hinges on the invisible physical realities of the experimental setup – like the way a small magnet spins under a beaker.
By paying attention to these overlooked details chemists at all levels – from students to professors – can avoid misleading results and make better, more reproducible discoveries, ultimately leading to better science. The tools we take for granted may have more impact than we think. Because in chemistry, how you stir things, can change the outcome.
References
1 VA Cherepanova, EG Gordeev and VP Ananikov, JACS Au, 2025, DOI: 10.1021/jacsau.5c00412










No comments yet