Carbon–carbon bond breaking reaction might make chemists rethink how they build molecules
A reaction that deconstructs rings could change the way chemists think about carbon–carbon bonds: as potential functional groups rather than inert structures. ‘I think that the next frontier is really thinking how to break carbon–carbon bonds that lie at the core of organic compounds to make value-added products,’ says Richmond Sarpong from the University of California Berkeley, US, who led the work.
There are hundreds of carbon–carbon bond forming reactions, many of which – like Grignard reactions or cross couplings – are organic chemists’ bread and butter. Researchers have even started to use artificial intelligence to sift through the millions of ways to connect carbons to nitrogen, oxygen or other carbons.
However, ‘a lot of late-stage diversification is based on C–H functionalisation, which functionalises molecules on their periphery,’ says Sarpong. ‘If you want to achieve more diversification, you need to think about the core of these molecules, which consists of carbon–carbon bonds.’ Since it is very difficult to predict how chemical structure relates to biological activity, freely exploring chemical space is important in drug discovery.
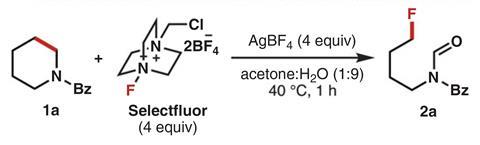
Sarpong’s team has now added the first deconstructive fluorination to synthetic chemists’ toolbox. The reaction breaks open cyclic amines and, at the same time, adds a fluorine and an aldehyde to the ends of the new linear molecule. There are some reactions like ozonolysis and metathesis that break double bonds and others that open small strained carbocycles. But this is the first strategy to work on single bonds in six, seven and eight-membered rings. ‘We are particularly excited about being able to make non-canonical peptides bearing fluorines,’ Sarpong says about the reaction on pipecolic acid derivatives.
‘It’s a very useful reaction and the conditions are straightforward,’ comments Joëlle Prunet, an organic chemist at the University of Glasgow, UK. ‘It’s the type of methodology I like, because it’s so simple but you’ve had to have the idea.’
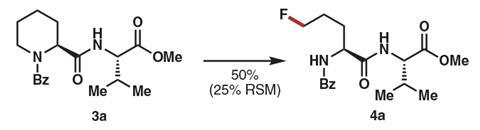
Although the reaction needs an excess of both the fluorine donor Selectfluor and a silver salt, ‘using four equivalents of it is probably cheaper than using 10 mol% of some palladium catalysts’, Prunet says. ‘I’m sure this drawback will disappear eventually and the yields are also going to be optimised, especially if it’s used by medicinal chemists or the pharmaceutical industry,’ she continues.
Sarpong’s team is now working on installing other halogens during the deconstruction. ‘This could then be used to extrude one carbon atom from a ring,’ he explains. ‘Making bonds is always going to be important, but we can now think about breaking bonds to make new ones.’
References
J B Roque et al, Science, 2018, DOI: 10.1126/science.aat6365














No comments yet