Elegant strategy that directly assembles 5–8–5 scaffolds from simple precursors paves way to a new library of bioactive compounds
5–8–5 ring systems are central to over 30 natural products, several of which have therapeutic activity. Despite its promising potential, drug-discovery programmes have largely ignored this particular ring system because it’s so difficult to make. A new synthetic strategy devised by scientists in the US could change all that.
The dicyclopenta[a,b]cyclooctene (5–8–5) system is an elegant carbotricycle comprising two 5-membered rings fused to a central 8-membered ring and is found at the core of many diterpenes, including fusicoccin. Diterpene compounds containing a 5–8–5 scaffold are often extraordinarily complex and as a result, quite tricky to synthesise. Current techniques to access these scaffolds for total synthesis rely on cycloaddition chemistry, and to prepare the precursors requires intense multi-step synthesis procedures involving transition metal catalysts and high temperatures.
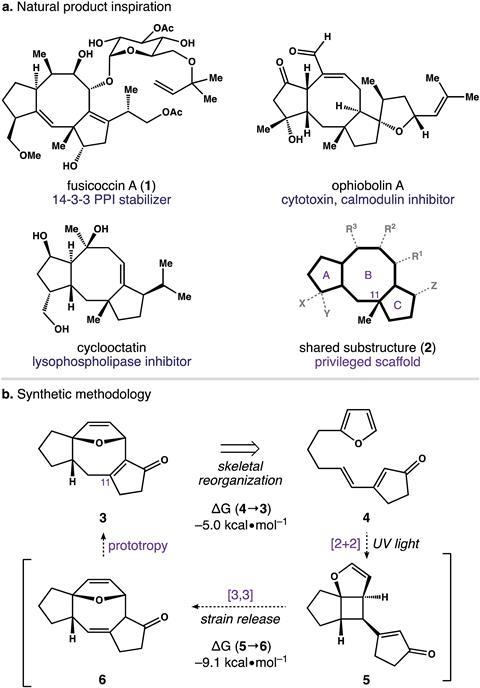
James Frederich and his team at Florida State University have now uncovered a modular strategy to access these complex ring systems in four steps. Their new strategy involves simple precursors and [2+2] photocycloaddition leading to a Cope rearrangement and a final isomerisation step to obtain the bioactive 5–8–5 carbotricycle core in gram quantities.
As with many synthetic methodologies, the route is not without its difficulties: ‘Our chemistry relies on UV-light to initially promote a [2+2] photocycloaddition. The rearrangement of the resultant cyclobutane to the desired 5–8–5 scaffold is quite efficient. However, in many instances the competitive photodecomposition of the initial cyclisation substrate is difficult to control,’ explains Frederich. ‘I’m new to the field of organophotochemistry; however, it has been my experience that small, seemingly insignificant changes in our photosubstrates can have a profound impact on the [2+2] photocycloaddition,’ he adds.
‘Compounds with 5-8-5 ring systems have a rich diversity of bioactivity but their extraordinary molecular complexity makes total synthesis challenging, which in turn, limits their representation in the drug discovery process,’ says Kay Brummond, an expert in natural product synthesis at the University of Pittsburgh, US. ‘The visual simplicity of this transformation will greatly assist in the implementation of this synthetic strategy to preparation of compounds possessing 5-8-5 ring systems.’ Brian Stoltz, a natural product synthesis expert at the California Institute of Technology, US, is also impressed by Frederich’s approach: ‘The combination of a [2+2] photocyclisation and a 3,3 sigmatropic rearrangement to accomplish a net [4+4] cycloaddition reaction is a beautiful combination of strategic design and tactical implementation. The researchers should be commended for their efforts!’
Frederich says his strategy will help identify new medicinal compounds and has plans to apply the methodology to explore how variants of this 5–8–5 scaffold function as stabilisers for protein-protein interactions in vivo: ‘These features improve access to this interesting chemotype and will hopefully aid in the identification/development of novel bioactive compounds.’
Correction: Comments by Kay Brummond were amended to more accurately reflect her views on 26 June 2018.







No comments yet