Photocatalytic method that upcycles aromatic polymers could breathe new life into rubbish

US scientists have developed a photocatalytic C–H fluoroalkylation approach for functionalising waste plastics. The method generates polymers that retain the favourable thermomechanical properties of the original plastics but with larger surface energies and new functionalities, leading to a variety of potential uses.
Plastics comprising aromatic polymers, which have high thermal and chemical stability, are hard to recycle. Moreover, recycling typically downgrades the quality of plastic, merely delaying it from entering landfill, being incinerated or exacerbating plastic pollution.
Frank Leibfarth and colleagues at the University of North Carolina at Chapel Hill, inspired by the unique and useful properties of fluorinated polymers such as Teflon, have come up with a way to upcycle commercial aromatic polymers via C–H fluoroalkylation.
Using a ruthenium catalyst to generate electrophilic fluoroalkyl radicals under mild conditions, the team chemoselectively functionalised commercial polymers such as polystyrene, to generate fluoroalkylated polymers. These polymers retained the attractive thermomechanical properties of the starting plastics, but had new properties such as high surface energy. The method avoided unwanted side reactions, such as crosslinking or chain scission, which can cause products to lose their beneficial properties. And it allowed the researchers to tune the new material’s properties by changing reagents and concentrations, as well as chemically diversify the products by incorporating new functional handles.
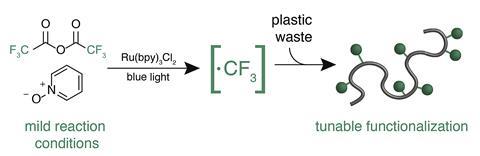
The high surface energy of the fluorinated products opens the door to a range of industrial applications, such as coatings to decrease surface tension and friction. Leibfarth is particularly excited that the polymers could serve as compatibilisers: ‘Compatibilisers allow you to gain both of the beneficial properties of two immiscible polymers in a single material, and that’s especially important in recycling waste streams. There are not a lot of ways to go from commodity polymers to compatibilisers like this.’
Brent Sumerlin, a polymer chemist at the University of Florida, US, sees the potential of the approach. ‘There’s a clear awareness in the polymer community that commodity plastics can be viewed, not just as nuisance waste products, but rather as abundant raw materials to create valuable new products. This is a clever approach to fluorinated materials and is made even more attractive by relying on photocatalysis to drive the process.’
Leibfarth hopes the method will create an economic incentive towards recycling waste plastics. The group is now working on developing cheaper catalysts to improve the process, and further exploring the potential applications of the resulting polymers.
References
This article is open access
S E Lewis, B E Wilhelmy Jr and F A Leibfarth, Chem. Sci.¸ 2019, DOI: 10.1039/c9sc01425j












1 Reader's comment