Relativistic effects stabilise square planar platinum(0) complexes
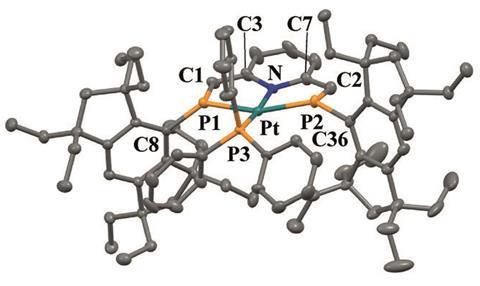
Normally, metals with 10 electrons in their outer d orbitals show tetrahedral geometry. However, a new platinum(0) complex adopts an unusual square planar geometry due to relativistic effects in orbital hybridisation.
A team of Japanese researchers led by Fumiyuki Ozawa at Kyoto University synthesised three coordination compounds bearing the same phosphines but different metallic centres – nickel, palladium, and platinum. Although nickel and palladium adopted the expected distorted tetrahedral geometry, to their surprise platinum didn’t. The platinum complex was completely flat.
Square planar complexes of d10 metals are quite rare, and they often require stabilisation by anionic ligands. But Ozawa’s team isolated their flat platinum complex using only uncharged phosphines. X-ray studies confirm its planarity.
The team also conducted computational studies to explain the extraordinary structure. Results suggest that relativistic effects induce an uncommon hybridisation of platinum’s 5d and 6s orbitals that favours the square planar geometry.
References
K Takeuchi et al, Angew. Chem., Int. Ed., 2016, DOI: 10.1002/anie.201609515














No comments yet