Gold nanoparticles functionalised with amino acid polymer inhibit the growth of amyloid fibres associated with neurodegenerative disease
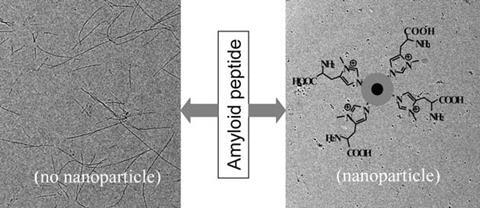
Nanoparticles coated in an amino acid polymer have been found to prevent the formation of amyloid fibrils – incorrectly folded protein fibres associated with the development of neurodegenerative diseases such as Alzheimer’s, Parkinson’s and Huntington’s.
When an amyloid protein folds abnormally, an insoluble fibre called a fibril is formed. If several fibrils clump together they create a plaque in the brain. By stopping fibrils forming, researchers hope to develop new treatments for these neurodegenerative diseases.
Nikhil Jana and colleagues at the Indian Association for the Cultivation of Science in West Bengal functionalised the surface of gold nanoparticles with five different polymer coatings based on different chemical building blocks. Jana’s team then mixed the nanoparticles with amyloid protein fragments and used electron microscopy and fluorescence analysis to monitor fibril formation. No fibril formation, or fibrillation, was observed when a high concentration of histidine polymer-coated nanoparticles was used. ‘This work shows that nanoparticles can be designed with appropriate surface chemistry to completely inhibit amyloid fibrillation,’ says Jana. As they increased the concentration of histidine nanoparticles, the team noticed that fewer fibrils formed. This suggests that the degree of fibril formation can be tuned by varying the nanoparticle dose.
Previous studies have shown that molecular histidine interacts with amyloid protein, but only very weakly, so doesn’t prevent fibril formation. When histidine is prepared as a polymeric nanoparticle coating, however, it can bind strongly to the growing fibril at multiple sites. This leads to short fibrils that cannot combine to create a full, mature fibril.
Control experiments with other polymer coatings reveal the properties of histidine that make it ideal for this application. To provide efficient inhibition of fibril growth, a coating must have both a positive and negative surface charge, and a weakly hydrophobic functional group. Histidine ticks all these boxes, producing a coated nanoparticle with promising properties. Further work is needed to develop a biodegradable nanoprobe and a strategy to deliver this into the brain. Even so, Jana says that this investigation has demonstrated the impact nanoparticles could have on the prevention and detection of neurodegenerative diseases.
‘These results reveal the high level of sensitivity of protein aggregation to external factors,’ explains Tuomas Knowles, who studies protein self-assembly at the University of Cambridge, UK. ‘Nanoparticles have significant potential as modulators of this important [fibrillation] process.’












No comments yet