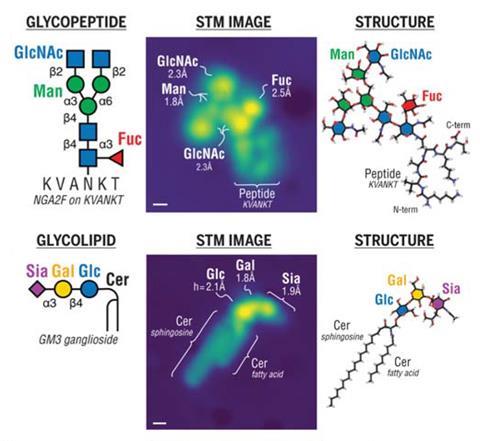
Scanning tunnelling microscopy has enabled researchers to directly image important sugar molecules attached to lipids and proteins. The experiments provide a picture at the single-molecule level of the sequences and locations of glycans bound to important biomolecules, offering new insight into the role they play in biology.
Glycans are carbohydrates that play critical roles in almost all biological processes. But despite their abundance in cells, these molecules are notoriously difficult to study. Now, a team of researchers from across Europe has used microscopy techniques to reveal new details about how glycans bind to proteins.
The team, which was co-led by Kelvin Anggara from the Max-Planck Institute for Solid-State Research in Germany, used electrospray techniques to deposit glycan-decorated protein and lipid molecules, known as glycoconjugates, onto copper and silver surfaces. This allowed them to image the molecules directly using a scanning tunnelling microscope.
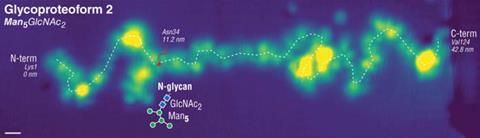
The experiments enable the researchers to determine which individual monosaccharides are present in the glycan chains and learn more about their orientations and where exactly they are attached on a protein backbone.
In a ‘tweetorial’ on the work, Anggara showed how the technique was used to interrogate all of the oxygen-linked glycans on a fragment of a human mucin protein. The research team described this as ‘one of the most complex glycoproteins in biological systems’ and it is also a promising cancer biomarker.
Out today in @ScienceMagazine: Seeing glycans attached to proteins and lipids - at the *single molecule level*! 🎉🎉🎉 https://t.co/H2110fWY6m#ScienceResearch #glycotime #teammassspec
— Kelvin Anggara (@vixklen) October 12, 2023
My 1st #tweetorial🧵(1/n) 👇👇👇 pic.twitter.com/4OzmBWwRfl
The team hopes that, with further development, the technique could be used to help identify unknown glycoproteins and glycolipids, helping to provide new understanding of the important role these molecules play in biology.
References
K Anggara et al, Science, 2023, DOI: 10.1126/science.adh3856

















No comments yet