Leveraging the ability of living systems to evolve and overcome complex problems, scientists in the US have created strains of Escherichia coli capable of anaerobically producing n-butanol, 1,3-butanediol and 4-hydroxy-2-butanone. The team identified two gene loci responsible for altering the flow of carbon from acetyl coenzyme A towards valuable C4 commodity chemicals instead of biomass. Matthew Davis who contributed to the research as a graduate student in Michelle Chang’s lab at the University of California, Berkeley, describes the work as ‘an example of the classic experimental strategy of using evolution to efficiently find solutions to complex biological problems.’
Living systems hold tremendous potential for synthesising desired carbon compounds, and their use of building blocks sourced from renewable sources could help shift chemical production away from petrochemical feedstocks. However, redirecting biological pathways away from their natural products, particularly to more complex carbon-based molecules, is difficult. ‘Cells have evolutionary and growth objectives that are diametrically opposed to producing a single product,’ explains Michael Jewett, a professor of chemical and biological engineering at Stanford University, US, who was not involved in the research.
Chang and her team used techniques including natural adaptive evolution, gene knockout, enzyme screening, metabolomics and genetic selection to improve the yields of n-butanol, 1,3-butanediol, and 4-hydroxy-2-butanone from 11–20% to near quantitative yields in E. coli. These three industrially relevant C4 chemicals can be further dehydrated to produce 1-butene, 1,3-butadiene and methyl vinyl ketone, respectively.
Their strategy relied on using product titre as a marker for quantitatively assessing genetic traits and creating successive generations that, in the end, feature large shifts in the regulatory and metabolic network of the cells. ‘We engineered a system where cell growth and product formation were linked, which provided an evolutionary advantage to cells that made more product, and thus grew better,’ outlines Davis.
Adaptive evolution helped the team uncover a smaller number of powerful mutations, as opposed to synthetic mutagenesis methods, explains Davis. ‘For example, we briefly explored the chemical mutagen ethyl methanesulfonate, and although this did produce higher performing strains, their genomes contained 50–150 mutations, the vast majority of which are likely to be neutral for performance.’
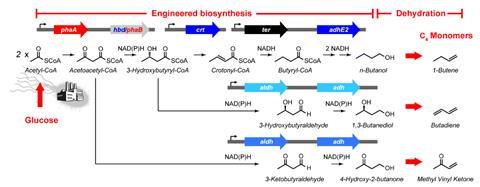
The team sequenced the genomes of a total of 31 isolated strains from three independent selections for n-butanol, 1,3-butanediol and 4-hydroxy-2-butanone production to identify genomic mutations. the team found that mutations in two gene loci, pcnB and rpoBC, were sufficient to enable the needed shifts in carbon flow.
Davis says several of the techniques they used, such as whole-genome sequencing and metabolomics allowed them to probe microbial systems ‘more comprehensively, gaining insight into both how they are regulated as well as suggesting engineering strategies to optimise biological systems for industrial applications’. ‘The generalised strategy of transcriptional rewiring should … be a fruitful area for future studies,’ he adds.
‘Their work sets the stage for scale-up opportunities to transform the bioeconomy,’ says Jewett. ‘Most of the things around us, from paint to shoes, are created from carbon-based chemicals produced from fresh fossil sources that release carbon dioxide into the atmosphere. Biomanufacturing offers a different, more sustainable approach to these products, shifting chemical synthesis towards use of above ground carbon.’
‘Over time I believe that fermentation can become a viable manufacturing method for many industrial products, but there is still a long road ahead,’ concludes Davis. ‘There’s so much to consider, but the drive to build something new is what motivates us day by day.’
References
This article is open access
M A Davis et al, Chem. Sci., 2023, 14, 11718 (DOI: 10.1039/d3sc02773b)













No comments yet