A combination of electrochemistry and organic catalysis transforms alkenes into hard-to-make vicinal diamines
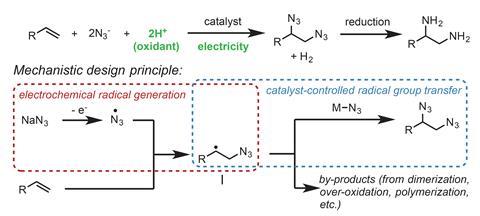
Organic chemists in the US have zapped alkenes with an electrical current to make vicinal diamines. These useful structures appear in many bioactive molecules, such as the antiarrhythmic lidocaine.
To make vicinal diols, there are simple reactions to install two hydroxy groups on either end of a carbon–carbon double bond. Equivalent methods for making 1,2-diamines, however, are lacking: they use exotic nitrogen reagents or toxic heavy metals like osmium. Often, they are also incompatible with sensitive functional groups in most complex drugs or natural products.
Song Lin’s team from Cornell University has now combined electrochemistry with organic synthesis into a cheap and easy method that adds two nitrogen groups to either side of alkenes. The reaction needs only two reagents: sodium azide – the nitrogen source – and manganese bromide, which acts as a nitrogen transfer catalyst.
The electrochemical reaction, which can be powered by two AA batteries, produces azide radicals. One of these radicals attacks one side of the alkene’s double bond. The catalyst then transfers a second azide to the other side of the double bond. The resulting vicinal diazide is converted into the diamine using one of the many reduction protocols, such as hydrogen and a palladium catalyst.
The researchers found the reaction suitable for acyclic and cyclic alkenes with all sorts of substituents, including those sensitive to oxidation (eg alcohols) or nucleophilic substitution (eg esters). Even tetrasubstituted alkenes, which often fail to react because they are so sterically crowded, were converted into diazides in over 80% yield.
Lin’s team hopes that their discovery will allow chemists easier access to diamines, using simpler precursors and cheaper reagents.
References
N Fu et al, Science, 2017, 357, 575 (DOI: 10.1126/science.aam9949)





![Top view of the single-crystal X-ray diffraction structure of a dithienothiophene (DTT)-bridged [34]octaphyrin](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/380x253/3/6/2/132362_Bicyclic-Baird-type-aromaticity-fig-1-c.jpg)









No comments yet