Chemists in the UK and Switzerland have synthesised the cyclocarbon molecule C16 and obtained the first proof of its anti-aromatic character. The rings are a new form of the element, and more novel cyclocarbons are on the way.
In 2019, researchers at Oxford University and IBM Research Europe – Zurich reported the on-surface synthesis of a new allotrope of carbon, C18 – a circular polyyne of 18 carbon atoms linked by alternating single and triple bonds. The molecule is also doubly aromatic, thanks to its two perpendicular π systems – one in the ring plane and one out of the plane.
Now the groups of Harry Anderson at Oxford and Leo Gross at Zurich have taken the carbon-ring concept a step further by adding a more challenging candidate: a ring of 16 carbon atoms.1 Unlike C18, which follows the Hückel rule for aromaticity with 4n+2 atoms, the C16 molecule is predicted to be doubly anti-aromatic, and thus destabilised by ring effects. Before the successful synthesis, it was not known whether it was possible to make such a molecule and keep it stable for long enough to study its structure and electronic properties.
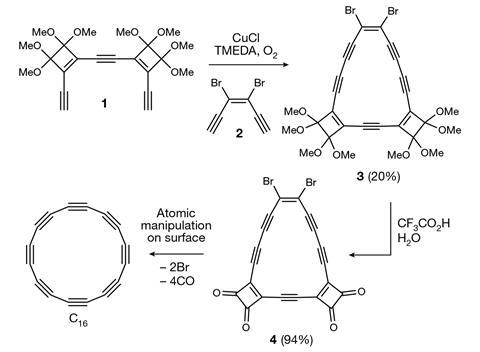
‘The challenge with making C16 was mainly the instability of the potential precursors, which are also anti-aromatic,’ Anderson explains. The macrocyclic precursors contain several ‘masking’ groups that stabilise the molecule. These groups can then be converted to alkyne connections by eliminating the masking substituent. An initial attempt to use only carbon monoxide masking groups analogous to the precursor used for C18 turned out to be too unstable to survive the logistics of the Oxford–Zurich collaboration. The successful protocol involves a macrocycle with four carbon monoxide and two bromine substituents, which is then deposited on a sodium chloride substrate. With a tunnelling microscope, the Zurich group targeted currents of a few picoampere to specific reaction sites, driving up the voltage to eject the bromines at around 1.3 V and then the carbon monoxide groups at around 3 V.
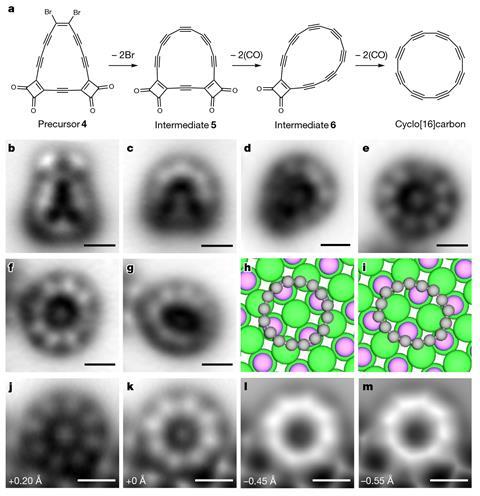
Atomic force microscopy confirmed the ring’s polyyne structure of alternating single- and triple-bonds. The researchers were also able to analyse the distribution of electrons in the molecular orbitals both for the neutral C16 molecule and for its negatively charged ion using Kelvin probe force microscopy and scanning tunnelling microscopy. These analyses and calculations confirm that the electronic ground state of the molecule is indeed doubly anti-aromatic.
The molecule therefore opens new opportunities for experimental exploration of these theories. ‘Organic chemists love to discuss and debate the concept of aromaticity, and the remarkable synthesis of the doubly anti-aromatic C16 is bound to raise these debates to new heights,’ remarks Rik Tykwinski from the University of Alberta, Canada.
The cyclocarbon family may soon grow even larger. Wei Xu’s group at Tongji University, China, has three papers currently under review describing the syntheses of cyclocarbons C6, C10, C12, C14 and C20.2,3,4 These cover the entire size range over which these rings are now believed to be sufficiently stable for studies.
With a whole array of new molecules to experiment with, making them react with each other could be the next step. ‘The next breakthrough in this field could be the discovery of ways to control the reaction of cyclocarbon molecules with each other to generate extended carbon allotropes,’ Anderson says.
References
1 Y Gao et al., Nature, 2023, DOI: 10.1038/s41586-023-06566-8
2 W Xu et al., Research Square (preprint), 2023, DOI: 10.21203/rs.3.rs-3411973/v1
3 W. Xu et al. Research Square (preprint), 2023, DOI: 10.21203/rs.3.rs-3411973/v1
4 L. Sun et al., Research Square (preprint), 2023, DOI: 10.21203/rs.3.rs-2616838/v2



![Cyclo[48]carbon [4]catenane](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/159x106/4/6/3/542463_indexady6054_articlecontent_v2_18june3_70174.jpg)

![Cyclo[48]carbon [4]catenane](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/380x253/4/6/3/542463_indexady6054_articlecontent_v2_18june3_70174.jpg)







No comments yet