Meet the biphen[n]arenes, a new family of macrocyclic arenes
Macrocyclic scaffolds have been hugely influential in supramolecular chemistry and now scientists in China have synthesised a new addition to this pool of chemical building blocks.
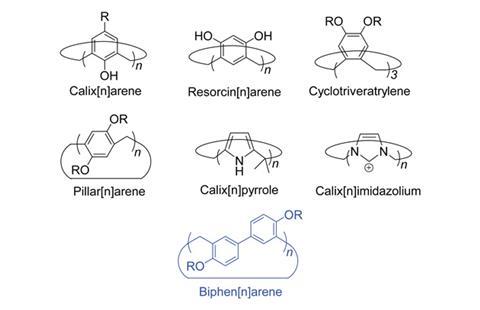
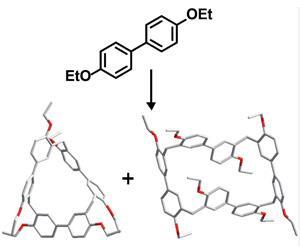
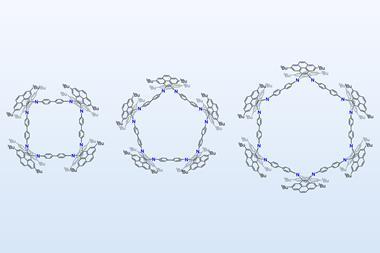
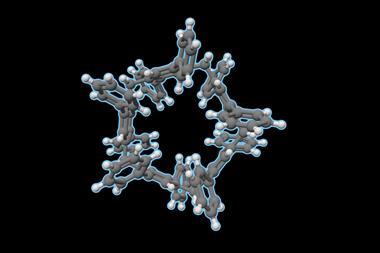
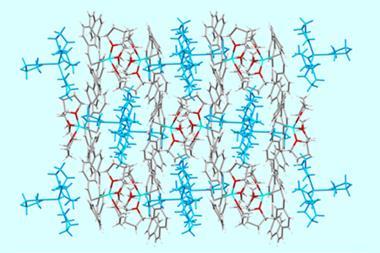
The biphen[n]arene macrocycles, created by Chunju Li of Shanghai University and colleagues, are based on 4,4’-biphenol and are reminiscent of popular phenol and biphenol based macrocycles such as calix[n]arenes, resorcin[n]arenes and pillar[n]arenes. The biphen[n]arenes are p-electron rich, and like many of their predecessors can complex a range of cationic and neutral p-electron deficient guests within their cavity depending on their conformation. However, they have very distinctive geometries, including a triangular prism and a molecular box, which sets them apart from common macrocyclic arenes based on mono-benzene units.
The biphen[n]arene hosts are easy to prepare in a one-step reaction with commercially available reagents. Their per-hydroxylated forms are also readily accessible and could be useful in the future for adding further functionality to these structures. Considering the current interest in pillar[n]arene chemistry and the tremendous successes of other macrocyclic arenes, could biphen[n]arenes be the next popular scaffold in supramolecular chemistry?
References
This article is open access. Download it here:






No comments yet