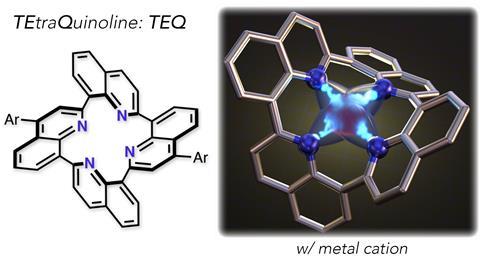
The nitrogen macrocycles family has welcomed a new member – tetraquinoline. Researchers in Japan have reported the first-ever synthesis of this porphyrin-like structure, which shows strong affinity to certain metals, despite its non-planar structure. Tetraquinoline complexes could find applications in catalysis, pH probes and sensors, based on preliminary studies.
Nitrogen macrocycles consist of linked units of heterocycles. Porphyrin, for example, features four modified pyrrole units, and is common in nature, found in colourful complexes in chlorophyll and haemoglobin, among other structures. For years, chemists have varied the units and linkers to form other macrocycles, each with different properties and possibilities. However, tetraquinoline remained a missing link – until now.
Unlike most nitrogen macrocycles, tetraquinoline exhibits a three-dimensional structure – it’s shaped like a saddle. This unusual structure was confirmed by both computational simulations and single-crystal x-ray diffraction. Researchers report that a key step towards constructing tetraquinoline was the formation of a cyclic diamide intermediate that’s already equipped with the 16 internal atoms of the final product. The subsequent synthesis of the remaining pyridine rings followed a previous procedure, optimised to combine amides and nitriles into heterocycles in one single step.
Despite the twisted structure – and the slightly unaligned nitrogen atoms – tetraquinoline successfully coordinates cations to create complexes. Researchers prepared combinations with first-row metals and elements such as sodium, potassium, magnesium and calcium – all abundant in cells. Additionally, tetraquinoline yielded a strong sky-blue fluorescence when protonated or combined with zinc. This could lead to sensitive and selective zinc probes, with applications in biochemistry and life sciences, as well as accurate pH meters.
Tetraquinoline–iron complexes have shown catalytic activity in relevant redox reactions, with low catalyst loadings. According to the Japanese team, other tetraquinoline-based compounds could find further benefits in reactions like carbon dioxide reduction and water splitting, much like other pyridine-based complexes.
References
W Xu et al, J. Am. Chem. Soc., 2023, DOI: 10.1021/jacs.2c12582













No comments yet