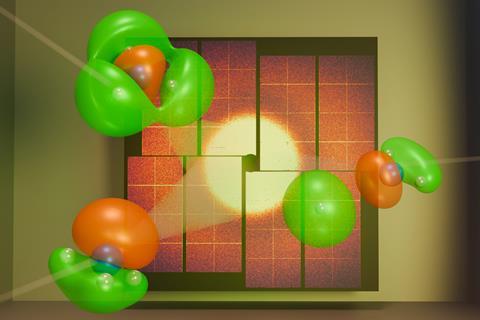
Chemists at a giant x-ray laser in the US have studied a reaction in the most extreme detail yet, watching individual valence electrons as ammonia dissociates. ‘For the first time here, we were able to track just a single valence electron,’ says Ian Gabalski, a PhD candidate at Stanford University in California. This is likely the first of many such experiments that will help scientists to better understand the processes of making the chemicals that are important to us.
Valence electrons are an atom’s outermost electrons and define its chemical behaviour. They ‘are shared between atoms, and so they basically drive all chemical reactions’, emphasises Gabalski. ‘They move on ultra-fast time scales, and they’re also covering very small distances.’ To measure them requires light pulses shorter than the time it takes for the electrons to move. The distance between peaks in the lightwave, better known as the wavelength, must also be similar to the size of the electron orbital. ‘That ends up being an x-ray,’ Gabalski says.
Gabalski was part of a team at the SLAC National Accelerator Laboratory in California led by Mike Glownia and Philip Bucksbaum. Together the researchers used ultraviolet laser light to trigger individual hydrogen atoms to separate from ammonia molecules. They then fired x-ray beams from SLAC’s Linac Coherent Light Source (LCLS) at the molecules after an infinitesimally small delay.
Gradually changing the length of the delays, the team studied the valence bonding electrons over a few hundred femtoseconds. For an idea of how short that is, there are 1015 femtoseconds in a second, whereas the universe is about 7 x 1015 minutes old.
Turning valence electrons off and on again
Researchers previously saw fleeting glimpses of changes in valence electrons in similar experiments. However, they studied more complex molecules, which threw up unexpected problems. ‘That short pulse of x-rays ends up taking a picture of all the electrons in your system,’ Gabalski explains. ‘Most of the electrons in the molecule are localised around the heavier atoms, and so they end up being the dominant contribution to the scattering.’
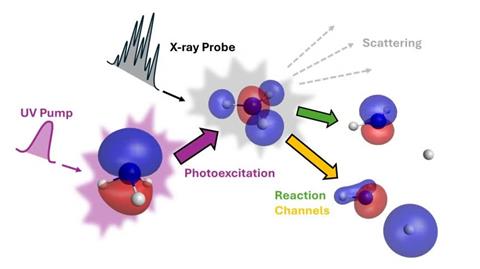
To find a system where this problem didn’t happen, the LCLS scientists worked with Nanna List at the KTH Royal Institute of Technology in Stockholm, Sweden, and the University of Birmingham, UK. List explains that such experiments have been motivated by researchers showing that it should theoretically be possible to make x-ray scattering measurements attributable to valence electrons.
List could use similar theoretical calculations to ‘decide on a system to target’, namely ammonia. These calculations were part of a detailed proposal the scientists used to apply for much-coveted time on the LCLS x-ray laser.
The scientists recorded the changes in angle of x-ray beams scattered by ammonia molecules. From that they could work out where the electrons keeping the atoms in the molecule together as it changes from a pyramidal to a planar shape were, before a hydrogen breaks off.
List’s theoretical models helped the LCLS team to work out what the valence electron was contributing, turning the effect of its presence on and off. Gabalski explains that their measurements accord with a process where the ultraviolet laser causes the valence electron to become much more delocalised. It is easier for hydrogen to dissociate from this state, which lasts for around 100 fs.
Adam Kirrander from the University of Oxford calls the choice of the simple target system clever, because it makes it ‘easier to separate the electronic rearrangements from the structural changes in the signal ’. ‘It is a beautiful example of the increasingly accurate mapping of time-dependent dynamics in molecules that is possible,’ he says.
References
I Gabalski et al, Phys. Rev. Letts., 2025, DOI: 10.1103/53h3-vykl














No comments yet