The slipperiness of a surface can be tuned by tweaking its molecular-scale roughness, researchers in Finland have shown. This helps to explain why water droplets can easily slide off hydrophilic surfaces if they are chemically homogeneous, and also opens up new possibilities for the design of low-friction surfaces – allowing the researchers to produce what they believe is the most slippery surface ever created. Such surfaces could be used in self-cleaning coatings.
The slipperiness of surfaces is sensitive to topographic heterogeneity or roughness and some studies have shown that this sensitivity persists all the way down to the molecular level. Nevertheless, droplets do not readily slide on most hydrophilic surfaces, with some exceptions such as those based on polyethylene glycol. ‘There are just a few reported, but [researchers] don’t really explain the origin,’ says Robin Ras at Aalto University.
Ras and colleagues decided to systematically study how water slippage varied with hydrophobicity by placing pristine hydrophilic silicon dioxide in a vacuum chamber and using vapour deposition to form a partial hydrophobic octyltrichlorosilane monolayer on the surface. By varying the deposition time, they created surfaces that ranged from nearly entirely hydrophilic to a patchwork of hydrophilic and hydrophobic islands to almost completely hydrophobic.
When they tested the slipperiness of the surfaces, they found that water slid easily off surfaces densely covered with the hydrophobic octyltrichlorosilane. But water droplets slid just as easily off highly hydrophilic surfaces with very little coverage with this chemical. At intermediate coverage, water droplets experienced much higher friction than either the principally hydrophilic or hydrophobic surfaces. The researchers then turned to molecular dynamics simulations to explain why.
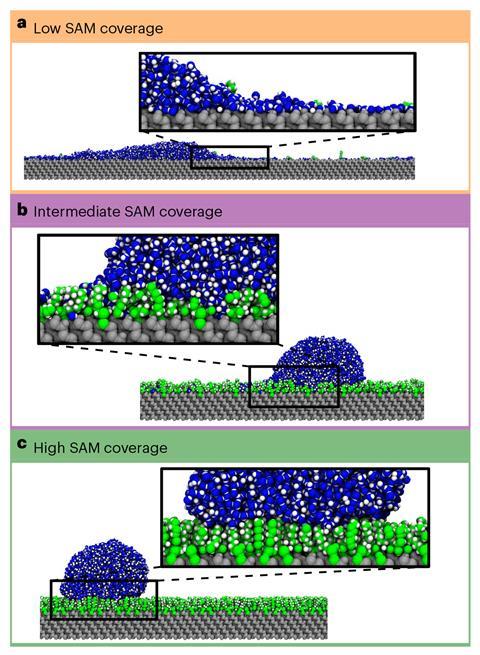
In the highly hydrophobic case, a water droplet touches the surface at a very steep angle, so only a relatively small area of the droplet made contact and slides off easily. This principle of texturing a surface to reduce a droplet’s contact area is well known and routinely exploited in superhydrophobic surfaces. The other cases surprised the researchers more. In the low-coverage, hydrophilic case, water spread out to form a thin film that covered the surface. Other water droplets formed large interfacial contacts with this layer, but as it was highly mobile it lubricated their passage across the surface. In the intermediate case, however, this hydrophilic surface effect was broken up by the hydrophobic islands. Instead of skating across the surface of a contiguous layer of water or skimming on a hydrophobic surface, water droplets were repeatedly puddling. ‘We actually had not expected this when we did the work, but looking back it fits very well with the idea that the heterogeneity is connected to the friction, so it’s kind of logical,’ says Ras.
The researchers extended their work to make a super-slippery superhydrophobic surface. They grew their hydrophobic monolayer on top of a layer of aluminium oxide-covered black silicon, which was textured at the micrometre scale to minimise the droplet contact area and create an exceptionally superhydrophobic surface. ‘The usual approach is to reduce the area at which the droplet can make contact with the surface by having as fine a structure as possible, but people have not really focused on tuning the surface chemistry,’ explains Ras. Here, however, the researchers combined both physical and chemical hydrophobicity. ‘The contact area is very small, and where it does contact the surface you have this lubricating effect,’ explains Ras. The result, they believe, is the most slippery surface ever created. Ras believes the approach is scalable and says he has had ‘many discussions with companies that have expressed interest’.
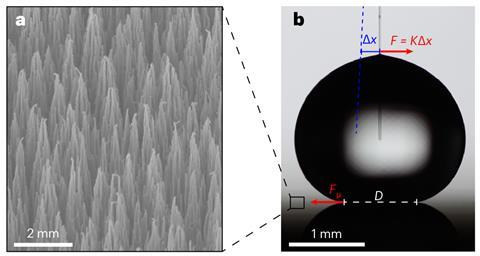
‘I think it’s an important paper,’ says Mathew Mate at SLAC National Accelerator Laboratory in the US, although he is curious about the researchers’ surprise. ‘It wasn’t counter-intuitive to me,’ he says. ‘I like the scientific research that’s done in this paper, and it really shows on a molecular level what I thought would happen.’ He also adds that, ‘if the superhydrophobic surface is the slipperiest one to date then that’s a very important discovery in its own right’.
References
S Lepikko et al, Nat. Chem., 2023, DOI: 10.1038/s41557-023-01346-3
















No comments yet