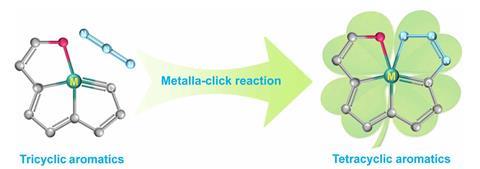
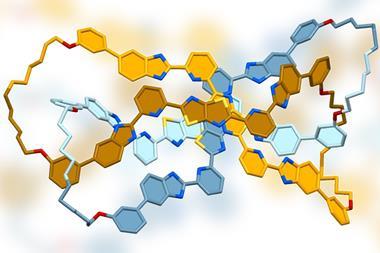
A molecule resembling a four-leaf clover – four fused rings connected by a central osmium atom – is the first member of a new class of aromatics. The molecule has been constructed using reactions that had remained unexplored for more than two decades.
To date, no molecule with more than three fused aromatic rings shared by one bridgehead atom had been made. But in what the authors of the new study call ‘never-ending pursuits for exploring aromatic molecular architectures’, they have now made the first compound in which a single metal atom connects four fused aromatic rings.
To make the compound, the chemists used a metalla-click reaction. A precursor containing three fused rings and an osmium–carbon triple bond reacts with an azide to form a five-membered ring, completing the four-leaf clover structure. Metalla-click reactions were first described 20 years ago, but had only ever been tested in two cases – one with a tungsten and another one using a molybdenum compound.
The osmium clover leaf survives being heated in air to 100°C for several hours – indicative of aromaticity, which tends to make compounds unusually stable. Other hints of the molecule’s aromatic character include the fact that it’s flat and a tell-tale downfield shift of its hydrogen atoms in the nuclear magnetic resonance spectrum. Calculations show that the osmium atom contributes two filled d orbitals to the overall aromatic π system.
The molecule absorbs light at different wavelengths, all the way from ultraviolet to near infrared. It also demonstrates a strong photothermal effect, meaning it converts infrared laser light to heat. Photothermal materials are being investigated as therapies for various diseases including cancer.
References
Z Lu et al, Sci. Adv., 2020, 6, eaay2535 (DOI: 10.1126/sciadv.aay2535)












No comments yet