
Hydrogen offers many advantages for clean energy storage, but storing it generally requires either a lot of pressure, a lot of heat or both. To avoid the enormous pressures needed to store pure hydrogen in manageable volumes, researchers have looked at hydrogen compounds. However, so far these approaches have suffered a catalogue of issues from high operating temperatures impeding the energy efficiency and stability of devices to low hydrogen storage capacity. Now researchers led by Naoki Matsui and Ryoji Kanno at the Institute of Science Tokyo in Japan have developed an electrolyte that allows high performance hydrogen storage.
There are currently two main approaches to solid state hydrogen storage. The more thoroughly researched is ‘thermodynamic’, whereby certain alloys absorb hydrogen and form hydrogen compounds that then break down and release hydrogen at higher temperatures. However, while some light-element metal hydrides, such as MgH2 and LiH have a high hydrogen storage capacity, temperatures of around 300°C are required to release the hydrogen. An alternative approach dates back to the work of Stanford University’s Robert Huggins, who in 1985 proposed that hydride ions could be electrochemically inserted into a metal using a liquid electrolyte and an applied voltage. However, once again the approach was complicated by high temperatures. Recent advances in solid state electrolytes may offer a way around this if adequate ionic conductivity and stability at lower temperatures can be achieved.
‘As we had been developing solid-state ionic conductors capable of transporting hydride ions, we recognised the potential for applying these materials to hydride driven electrochemical hydrogen storage,’ Matsui and Kanno told Chemistry World in an email. They had noted reports of superionic conductivity by combining ions of different sizes, and in pursuing a similar approach landed upon a compound combining barium, calcium and sodium ions: BaH2–CaH2–NaH.
Initial experiments used the electrolyte in a test system sandwiched between molybdenum current collectors and an acetylene black conduction additive, and TiH2 and titanium reference electrodes. This setup allowed them to determine the stable potential window of the solid electrolyte and Matsui and Kanno say that it is the best ever reported.
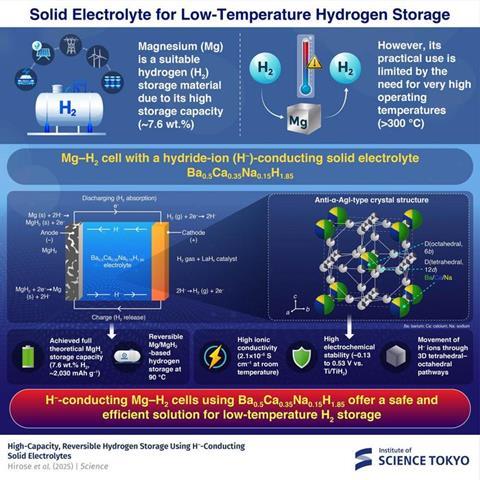
They also reported an unusually high hydride ion conductivity, which they attribute to the body-centred cubic crystal (bcc) structure of the solid-state electrolyte. ‘The energy barrier for ionic conduction is primarily determined by interactions with the surrounding framework,’ they explain. The bcc structure has a lower packing density compared with other common structures, ‘providing an open pathway for ion transport’. They also point out that the highly polarisable cations in the framework contribute to the high ion conductivity, as this reduces the repulsion between the cations and the hydride ions moving through.
To test the hydrogen storage capabilities directly they produced a cell with a magnesium hydride anode and a LaHx cathode. With this setup operating at 90°C and an applied potential of 0.12V, they were able to demonstrate the theoretical capacity of MgH2 over five cycles.
‘This paper, which demonstrates the new possibility of “hydrogen storage via electrochemical reactions”, represents an important milestone,’ says Genki Kobayashi, an inorganic chemist at RIKEN in Japan, who was not directly involved in the work. ‘I believe this work marks a turning point, and I look forward to further advances in the creation of new devices that utilise hydrides.’
Next, Matsui and Kanno plan to focus future work on developing solid electrolytes and electrode materials with higher ionic conductivity, alongside device designs with lower operating temperatures and improved energy efficiency.
References
T Hirose et al, Science, 2025, 389, 1252 (DOI: 10.1126/science.adw1996)

















No comments yet