Quantum simulations show that comets and other celestial bodies may have not only delivered building blocks for life to Earth, but also synthesised them on impact
Researchers from the US have discovered that exposing glycine solutions to sudden heat and pressure – 3000K and 48GPa to mimic a comet impacting Earth – can yield carbon-rich structures, including prebiotically-important nitrogen-containing polycyclic aromatic hydrocarbons (NPAHs).1
Glycine is a proteinogenic amino acid – one of 22 that are incorporated biosynthetically into proteins during translation. It is also the simplest amino acid and has been identified on comets such as 67P/Churyumov-Gerasimenko.2
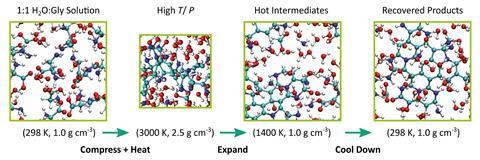
Now, Matthew Kroonblawd and colleagues at the Lawrence Livermore National Laboratory have used semi-empirical quantum simulations to understand what happens when glycine-rich icy materials crash into a planetary surface. The study modelled 10 solutions of glycine under hot and compressed conditions, followed by rapid expansion and cool down periods. They produced a variety of small organic products and NPAHs with different functionalities such as carboxyl, amine and alcohol groups, as well as aromatic heterocycles like furans and pyrroles. NPAHs are important precursors to nucleobases.
The researchers say their findings support an alternative pathway to circumstellar gas-phase radical reactions for creating NPAHs. It also verifies that prebiotic molecules carried by comets can not only survive an impact, but also react under such extreme conditions to produce a diverse series of organic products, adding to theories of how life came to exist on Earth.
References
1. M Kroonblawd, R Lindsey and N Goldman, Chem. Sci., 2019, DOI: 10.1039/c9sc00155g (This article is open access.)
2. K Altwegg et al, Sci. Adv., 2016, 2, e1600285 (DOI: 10.1126/sciadv.1600285)












No comments yet