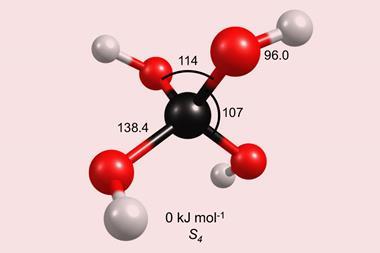
For the first time, methanetriol has been synthesised and characterised in the lab.
The molecule, which has three hydroxyl groups linked to a single carbon atom, is especially unstable under ambient conditions, because it tends to quickly react with other species or decompose and decay into more stable structures. The existence of methanetriol – conventionally known as orthoformic acid ‘had been hypothesised for decades’, according to lead author Ralf Kaiser from the University of Hawaii at Mānoa in Honolulu, US. ‘It took the unique environment of [simulated] interstellar ices to preserve this fragile molecule,’ he says.
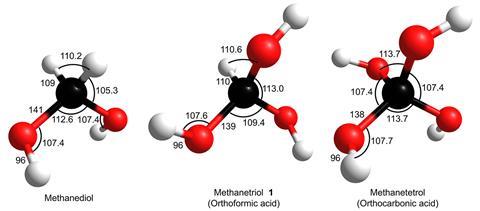
Back in 1880, German chemist Emil Erlenmeyer – famed for his flask – outlined a rule related to the stability of alcohols and tautomerism. It stated that compounds containing several hydroxyl groups on the same carbon atom aren’t stable. For methanetriol, ‘computational studies predict it’s driven to dehydration to form a carboxylic acid’, says Kaiser.
‘I can guarantee no one has seen a carbon in the wild with three [hydroxyl] groups!’ Chemistry World contributor Derek Lowe recently noted. However, in space the situation is different, which sometimes means unusual reactions occur.
‘[Our] experiments simulated the environment in a dense interstellar cloud,’ explains Kaiser. In such settings, temperatures reach 10K and ‘the only source of energy is the periodic passage of cosmic rays’.
To search for methanetriol, Kaiser and collaborators created interstellar ices in the lab made of a mixture of solid methanol and oxygen, which were then exposed to energetic electrons simulating the effects of cosmic rays. These frigid, high-energy chemical conditions then created methanetriol.
‘The synthesis and laboratory detection of methanetriol demonstrates this molecule, [which] isn’t stable under standard conditions on Earth, could be stable in space and perhaps even the upper atmosphere,’ says Olivia Wilkins, an astrochemist at the Nasa Goddard Space Flight Centre, US. ‘Studying such molecules pushes the boundaries of our understanding of how chemistry is influenced by physical environment,’ she adds.
To make methanetriol, researchers used an ultrahigh vacuum chamber and an ‘unfathomably cold’ piece of silver, kept at about 5K, explains Wilkins. Upon the deposition of methanol and molecular oxygen, ‘the gases immediately condensed [as solids] onto the cold silver’. Additionally, these unique conditions contributed to the isolation of methanetriol in two main ways. Firstly, the vacuum ensured the highly reactive methanetriol molecule couldn’t find anything around to quickly combine with. Moreover, the ultracold temperatures mean there’s less energy available and, therefore, reduced reactivity. The uncommon conditions and exceptional radiation required to prepare methanetriol suggest it could crop up in comets, typically abundant in methanol.
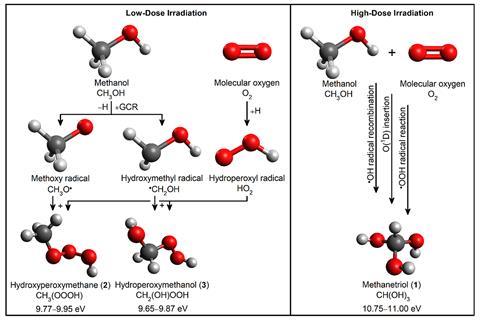
Slowly warming up the ice allowed the molecules in the sample to sublimate into a gas, which was gently ionised and characterised by mass spectrometry. ‘The presence of specific signatures predicted by computational simulations [corroborated] researchers had indeed made methanetriol,’ Wilkins says. Other experiments, including isotopic substitution studies, further confirmed methanetriol’s molecular formula.
‘Interstellar space has a lot of weird molecules that are not stable on Earth,’ comments Wilkins. ‘This makes the field [of astrochemistry] exciting, but also presents a challenge,’ she adds. Predicting the presence of ‘impossible molecules’ in space and the upper atmosphere requires an impressive imagination. For Kaiser, the importance of this discovery is also deeply symbolic. ‘The chemistry of the interstellar medium is more exotic and a continual source of new discoveries in chemistry,’ he says.
References
JH Marks et al, J. Am. Chem. Soc., 2024, DOI: 10.1021/jacs.4c02637















No comments yet