The tip of a scanning tunnelling microscope (STM) has been used to precisely switch between three possible isomers of a single molecule. The approach offers a new way to manipulate the configuration of 3D molecules using mechanical force and could find applications in the field of molecular machines.
Small structural variations often lead to significant differences in the chemical and physical properties of molecules, so controlling isomerisation at the single-molecule level is crucial in areas such as catalysis or drug synthesis. Scanning probe microscopy is a powerful technique for studying on-surface isomerisation and several strategies involving light and electrical activation have been proposed. Although using mechanical force to trigger the process offers additional benefits, advances in this area are still limited.
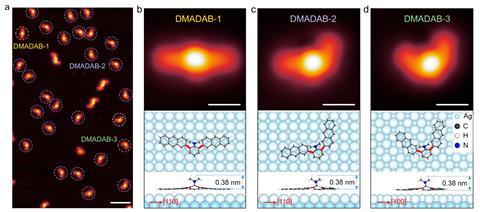
Researchers in China and Germany have now discovered a new way to induce reversible isomerisation of a single molecule on a silver substrate using an STM tip. ‘Mechanical force can be applied to specific atomic sites of the molecule, offering unprecedented control over the chemical process and products,’ points out Hong-Jun Gao of the Chinese Academy of Sciences. ‘In our study, we designed and synthesised a 3D, butterfly-shaped molecule called N,N-dimethylamino-dianthryl-benzene.’ Gao explains that when this molecule is placed on a substrate, the two anthryl groups are in-plane, forming the butterfly’s ‘wings’, whereas the dimethylamino group is located out-of-plane, acting as the ‘head’. This structure is important to ensure asymmetric interactions between the molecule and the tip, he says.
‘When the tip approaches the molecule, it interacts with only one of the wings due to the steric hindrance coming from the head of the butterfly. Inversely, when the tip is retracted from the molecule, the wing interacting with it is pulled away from the substrate while the other one stays attached. Upon further retraction of the tip, the molecule eventually detaches from it and falls on the surface with one of the wings completely rotated to form another isomer.’ The researchers found that without the out-of-plane group, the isomerisation process was blocked. They carried out molecular dynamics simulations and control experiments to confirm this mechanism. ‘Our method can be extended to other molecules,’ Gao says. ‘The key is to have a 3D molecular topology to allow different mechanical interactions with the tip.’
Thomas Frederiksen, who studies nanostructures and interfaces at the Donostia International Physics Center in Spain, notes that the study contributes to understanding possible strategies for manipulating single molecules on surfaces. ‘Such a level of control is of fundamental importance and can, for instance, help towards the design and operation of molecular machines,’ he says. ‘These results exemplify and enrich the active research field of atomic-scale manipulation and control, which has advanced substantially over the last decades.’
Takashi Kumagai of the Fritz Haber Institute in Germany and the Institute for Molecular Science in Japan, who demonstrated the hopping manipulation of two hydrogen atoms in a porphycene molecule,2 mentions that force-induced switching also works for other systems, although in different ways. ‘Since different molecules have different properties, the mechanism cannot be exactly the same,’ he says. ‘But it should always be associated with a modification of the potential energy surface, which determines the chemical dynamics.’ He adds that although the results are not revolutionary regarding the measurement technology, examples of force-induced single-molecule switching are very limited. ‘Therefore, this paper is an essential piece to establish single-molecule mechanochemistry, which I believe is an important emerging field.’
References
1 J Qi et al, J. Am. Chem. Soc., 2020, DOI: 10.1021/jacs.0c00192
2 J N Ladenthin et al, Nat. Chem., 2016, 8, 935 (DOI: 10.1038/nchem.2552)

![An image showing a [2]catenane](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/380x253/0/0/4/504004_fja0c01757_0007.jpeg_49119.jpg)







No comments yet