Performing enzyme-photocoupled catalysis in gas-sprayed microdroplets can boost the processes’ efficiency, new research shows. The method enjoys the selectivity of enzymes whilst harvesting solar energy to produce fine chemicals, yet dodges some of the issues that have stopped enzyme-photocoupled catalysis from being scaled-up.
Enzyme-photocoupled (EPC) catalysis combines enzymes and photocatalysts to generate higher product yields than can be achieved with either catalyst alone. So far, researchers have only explored the concept in laboratories. As with all photochemical processes, photon-transport attenuation effects hinder its scale up. ‘In a lab if we carry out EPC catalysis in batch, light utilisation efficiency is low. So we came up with the idea of using microdroplets, which can readily enhance light utilisation,’ says Jun Ge from Tsinghua University in China, who led the work with Richard Zare at Stanford University in the US.
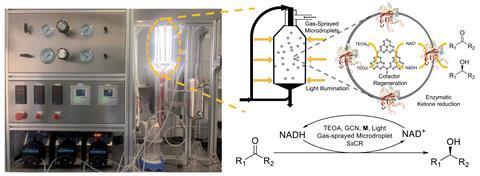
Converting bulk solutions into microdroplets reduces the optical path and increases light intensity. ‘We all know from water droplets in rain that we can see rainbows in the sky under the right conditions, so what is happening here is that [the droplet] collects the light and brings it to the backside,’ explains Zare. Also, the system benefits from the increased surface area of contact. ‘Imagine a one litre beaker of water and ask yourself what the surface area is. Now suppose you take the one litre beaker of water and you make it into droplets that are about 10 microns in size. If you work out the calculations, it turns out to be on the scale of a football field,’ continues Zare.
Usually, EPC catalytic systems suffer because radicals produced by the photochemical process deactivate the enzymes. However, in a gas-sprayed system, ‘the reaction happens on the surface of the droplets and the enzyme can be separated from the photocatalyst, so it avoids the deactivation of the [former] by the [latter],’ explains Ge.
When it comes to the actual reactor setup, Ge says it is rather simple. ‘An aqueous solution containing the enzyme and the photocatalyst is pumped into a nozzle, which sprays microdroplets with nitrogen gas into the reactor which has light shining around it. The product is collected in a receiver and both the enzyme and photocatalyst are reused.’
The EPC catalysis system was composed of a graphite-C3N4 photosensitiser, a rhodium electron transfer intermediate, the enzyme SsCR and the photocatalytic cofactor NADH to reduce ketones into chiral alcohols. Compared to a bulk process, the gas-sprayed EPC catalysed system uses solar energy much more efficiently while increasing both the reaction rate and product concentration.
‘Over the past decade, the special chemistry that occurs at interfaces, especially aqueous–air interfaces, has come to the fore,’ comments Graham Cooks, an expert in mass spectroscopy and electrospray microdroplets at Purdue University in the US. ‘The study raises intriguing questions regarding the implications of these findings for in vivo catalytic activity, where reactions at surfaces may play a role, which might be obscured by the bulk phase lock-and-key model that dominates explanations of accelerated reaction rates.’












No comments yet