Promethium’s coordination structure and bond length with oxygen have been characterised for the first time, filling one of the last gaps in our knowledge of the periodic table.

Promethium, element 61, is part of the lanthanides, which are widely used in modern technology. However, unlike its neighbouring elements, promethium is radioactive, highly unstable and does not exist naturally on Earth. It was only discovered in 1945, when it was created at Oak Ridge National Laboratory, US, by researchers working on the Manhattan Project, which developed the nuclear weapons that ended the second world war. Oak Ridge later played a key role in creating targets for making superheavy elements by nuclear fusion – a role recognised by element 117, tennessine, being named after the lab’s home state.
Since then, the difficulty and cost of working with promethium means that this element’s chemistry has largely been overlooked. While the element has some uses, such as in atomic batteries for space probes or cancer diagnostics, it remains one of the most mysterious elements on the periodic table.
Now, a team at Oak Ridge has revisited its first elemental discovery. The team used the lab’s nuclear research reactor to create the isotope promethium-147, which has a half-life of just 2.62 years, before the sample was chelated in a solution with diglycolamide ligands. The researchers then investigated the complex using synchrotron x-ray absorption spectroscopy, which allowed them to determine the properties of the complex, including measuring its bond length.
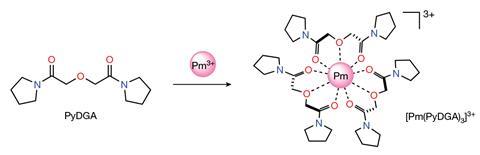
The team found promethium’s bond length with oxygen of 2.476Å fit perfectly with the trend seen in the other lanthanides, completing the experimental evidence for the contraction of lanthanides’ atomic radius – the size of the atom. As the atomic number of the lanthanides increases and the nucleus’s additional protons attract more electrons, these fill the 4f electron sub-shell. Usually, filled sub-shells are shielded from the nucleus’s charge by lower-energy electron shells. However, the 4f sub-shell is poorly shielded, which, along with relativistic effects governed by quantum mechanics, means the atomic radii of the lanthanides are smaller than predicted. This pattern becomes more pronounced as you move from left to right across the lanthanide series and the atomic number of the elements increases.
While this phenomenon is easy to measure in other lanthanides, promethium’s radioactivity and scarcity means it hadn’t been investigated before. ‘There are thousands of publications on lanthanide chemistry without promethium,’ says Santa Jansone-Popova, a scientist at Oak Ridge’s chemical sciences division, who co-led the study. ‘Scientists have to assume most of its properties. Now we can actually measure some of them … promethium was the last puzzle piece.’
The team’s work isn’t just about confirming theory, as metal–ligand bond lengths help define an element’s solubility in aqueous or organic solvents. ‘You cannot utilise all these lanthanides as a mixture in modern, advanced technologies, because you first need to separate them,’ adds Jansone-Popova. ‘This is where contraction becomes very important; it basically allows us to separate them.’
The team’s results are ‘seriously impressive’, says Conrad Goodwin, a Royal Society research fellow at the University of Manchester, UK, who specialises in f-block chemistry. ‘This is data that would otherwise be easy chemistry – simply making a Lewis acid-base adduct – except it’s with promethium-147, a radionuclide that simply wishes to not exist! That the authors have managed to do this is an amazing feat of experimental choreography … it’s satisfying to see the first truly complete series of molecular lanthanide complexes.’
References
DM Driscoll et al, Nature, 2024, 629, 819(DOI: 10.1038/s41586-024-07267-6)
















No comments yet