IF8 would have the highest ever coordination number in a neutral main group compound
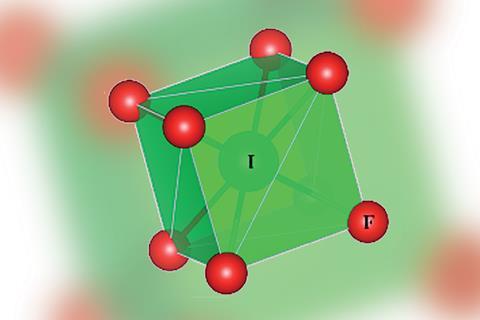
Neutral IF8, a new phase molecular iodine fluoride with an unusual cubical coordination geometry, is predicted to be stable at pressures above 260GPa, new research shows.
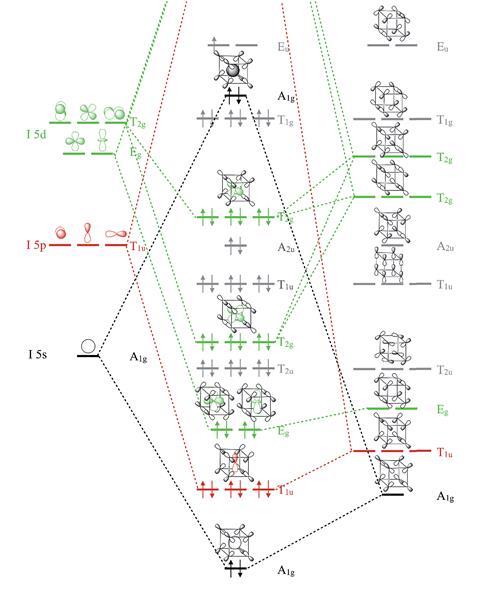
Researchers led by Guochun Yang of Northeast Normal University in China, Yanming Ma of Jilin University in China and Martin Rahm from Chalmers University of Technology in Sweden determined using first-principles calculations that the molecular crystal with R -3 symmetry would be metallic, with an electron-deficient electronic structure. Its quasi-cubic molecular configuration would be unique in main group chemistry and is distinct from the square antiprismatic structure of IF8−, common in main group chemistry; experimentally, scientists have never found a cubic ligand field in a molecule.
The iodine coordination number of eight is unprecedented; coordination numbers higher than seven in neutral main group compounds are hitherto unknown, except possibly in caged ions. The team predicted that iodine’s 5d band penetrates into the valence region and mixes with the 5s and 5p levels, therefore concluding that a valence expansion of iodine would occur.









No comments yet