Photoelectrochemical system theorised to produce high concentrations of liquid ethanol from carbon dioxide
Chemical engineers from the US have put forward a concept for a new type of artificial photosynthetic system to convert carbon dioxide into almost pure liquid ethanol fuel. It uses a saturated salt electrolyte, and, according to their calculations, the system would be capable of generating 15.27 million gallons of ethanol per year per square kilometre.
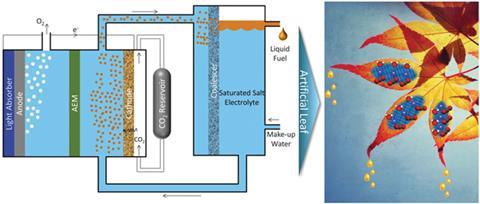
Ethanol is an attractive liquid fuel owing to its high energy density and market value per unit energy input. It is commonly produced by hydrating ethylene, or fermenting sugars derived from various agricultural byproducts.
Ethanol can also be made from carbon dioxide in a photoelectrochemical cell, which is attractive as it has the potential to support future energy demands whilst mitigating global warming. However, several challenges stand in the way of practical application. These include selectivity (production of a range of hydrocarbons as opposed to pure ethanol), membrane fuel-crossover losses (ethanol permeating to the wrong side of the cell and being converted into unwanted side products) and the high cost of purifying ethanol due to its high solubility in the aqueous electrolyte.
Alexis Bell and Meenesh Singh are experts in heterogeneous catalysis at University of California, Berkeley. They have proposed a photoelectrochemical cell that reduces the solubility of ethanol in the electrolyte through an effect known as salting out. By using an electrolyte supersaturated with caesium carbonate, the much stronger attraction between salt and water diminishes that of ethanol and water, which then form a microemulsion that can then be separated in a liquid–liquid extractor.
‘Salts are used extensively in industry to separate aqueous organic mixtures,’ explains Bell. ‘We have used the salting out effect and applied it to an artificial photosynthesis system that can naturally separate liquid fuels without requiring highly sophisticated membranes.’
A delicate balance
While the saturated salt electrolyte is the unique aspect of this design, a range of other parameters need to be carefully controlled to produce ethanol in sufficient concentrations for a microemulsion to form. Selectivity is imparted by a polycrystalline copper cathode and fuel-crossover is minimised by judicious choice of membrane in addition to the reduced methanol solubility. The current required to produce concentrations of ethanol high enough for a microemulsion to form is achieved by balancing the surface areas of the electrodes and the membrane that separates the two halves of the cell. By directly coupling the cell with the extractor and pumping the electrolyte in a circuit, this design integrates production with separation to theoretically produce more than 90% liquid ethanol.
‘Separating alcohols from water is a great challenge in artificial photosynthetic systems owing to the high solubility and crossover of alcohols,’ comments Hyunwoong Park, an expert in photo-energy conversion at Kyungpook National University, South Korea. ‘If experimentally proven, the proposed scheme is of great potential and highly applicable.’
Having established the feasibility of this design, Bell’s next step is to demonstrate it in a gas phase reactor. Balasubramanian Viswanathan, an expert in electrochemical energy at the Indian Institute of Technology, Madras, cautions that it remains to be seen whether this route will be cost effective. ‘Regardless, the impact of this design will be seen in almost all efforts of electrochemical energy conversion research,’ he says.
References
This article is free to access until 13 January 2016
M R Singh and A T Bell, Energy Environ. Sci., 2016, DOI: 10.1039/c5ee02783g












No comments yet