Researchers in Germany have developed a simple method for synthesising aluminium tris(fluorosulfate), a cheap and stable solid-state Lewis superacid.
Superacids are used to enhance the reactivity of other molecules by donating protons to them, making them much more reactive than normal so they undergo reactions they wouldn’t otherwise be able to. However, most superacids have drawbacks that limit their use, such as stability issues when making or storing them.
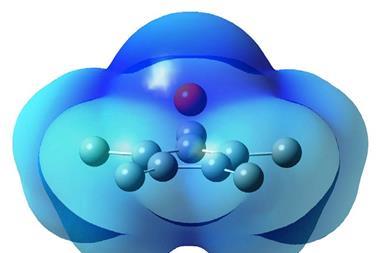
Aluminium tris(fluorosulfate) (Al(SO3F)3) has been known as a solid-state Lewis superacid for over 40 years, but its synthesis made it impractical for industrial use. By switching to a different starting material, researchers at the Free University of Berlin have developed an alternative preparation process that works in a single step and requires no work up to purify it. What’s more – unlike the original procedure – the new one doesn’t require the use of mercury to activate the aluminium in aluminium tris(fluorosulfate) before a reaction starts.
Adding fluorosulfuric acid to a frozen solution of trimethylaluminium and then warming the mixture to room temperature is all that is required to form aluminium tris(fluorosulfate), with only methane produced as a side-product. The polymeric product is stable and easy to handle and, combined with the relative simplicity and cheapness of the preparation method, should mean it becomes more cost effective to carry out superacid-catalysed transformations at large scales.





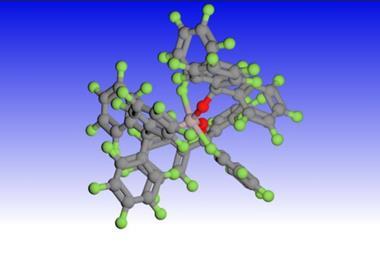
![A structure showing that the weakly bonded hydrogen of [P4H]+ sits on the edge of the P4-tetrahedron](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/380x253/7/8/5/138785_c8sc03023e-f5.jpg)








No comments yet