Photoredox catalysis, which harnesses the power of light to drive chemical reactions, has now gone asymmetric, allowing chemists to prepare valuable single-enantiomer products.
‘Photocatalysis exploits the energy of visible light to generate reactive radical species under remarkably mild conditions,’ explains Mattia Silvi, a photochemistry expert at the University of Nottingham, UK. ‘But the high reactivity of such species makes them particularly difficult to tame in the context of enantioselective processes.’ Many compounds exist in two mirror image forms known as enantiomers, which possess identical physical properties but interact differently with other ‘handed molecules’ such as amino acids and sugars. Access to the separate forms of these compounds is vital for the pharmaceutical industry, which must produce and test single enantiomers to guarantee the safety and efficacy of all new medicines.
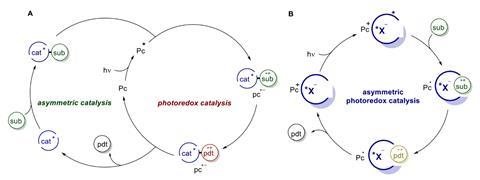
Typically, enantioselective reactions rely on orienting the substrates to form a specific product, but the unstable photocatalytic intermediates lack any means to exert this spatial control. Previous attempts to combine existing asymmetric methods, such as transition metal or enamine catalysis, with photoredox chemistry have met with only moderate success as they often require complex multi-catalyst systems which limit the reaction scope.
Instead, by introducing a chiral counteranion to direct the orientation of the positively-charged radical intermediate, Nobel laureate Benjamin List at the Max-Planck-Institute for Coal Research in Germany has developed a highly general method to control photochemical reactions. Known as asymmetric counteranion-directed catalysis, this ion-pairing approach has been widely adopted in other areas of organic catalysis, but a number of specific challenges exist for photochemical systems. ‘Photoredox catalysis goes asymmetric,’ is List’s simple takeaway message from his team’s work.
‘The radical cations formed are extremely acidic, so in the presence of a counteranion, they decompose by donating a proton,’ explains Sébastien Laulhé, a radical chemist at Indiana University–Purdue University Indianapolis, US. ‘ That means List has had to find a chiral counteranion that has a negative charge strong enough to electrostatically associate with the radical cation, but weak enough that it doesn’t decompose it.’
List’s team investigated the effect of a series of chiral anions on a photochemical [2+2] cycloaddition reaction, ultimately achieving an impressive 95.5:4.5 enantiomeric ratio on test substrates at −100°C using the highly confined imidodiphosphorimidate anion. Following this success, the team evaluated a range of substrates and commercial photocatalysts, demonstrating excellent functional group tolerance and broad compatibility with a variety of catalysts. ‘Any substrate that is activated by delivering an electron to a photocatalyst becomes positively charged and will associate with our anion, enabling the control of its reactivity and selectivity,’ says List. ‘There are a vast number of such reactions that could be handled this way, and [what’s] more, the inverse polarity, ie using a counteranion, is also possible. That is the beauty of the concept.’
List’s strategy addresses one of the longstanding problems in photoredox chemistry. ‘Merging enantioinduction with the tremendous versatility of photocatalytic reactivity provides an exciting approach towards enantioenriched motifs,’ says Mario Wiesenfeldt, a photocatalytic chemist at Ruhr University Bochum, Germany. ‘Given the high potential for generality, it could be worthwhile to develop a room-temperature version of this mode of enantioinduction. I would, however, be equally interested to see this idea applied to further photocatalytic transformations.’
The List group are keen to apply their asymmetric approach to traditionally racemic photochemical reactions and hope that this strategy opens up new and exciting possibilities for synthetic chemists across the board.
References
S Das et al, Science, 2023, DOI: 10.1126/science.ade8190














No comments yet