A new study has shown that precisely editing the ligands of a gold nanoparticle catalyst can alter the ratio of products formed by an organic reaction. With such a degree of control, this could prove to be a potent tool in the arsenal of synthetic chemists trying to manipulate increasingly complex reactions in pursuit of new molecules.
In heterogeneous catalysis, substrates bind and react at specific locations on the catalyst surface. A major aspect of understanding catalytic reactions is identifying these active sites. Changing them can affect the outcome of a reaction by altering the preference for one product over another.
If the product of a reaction is an active pharmaceutical ingredient, for example, it is important to avoid unwanted side products. Using a bespoke catalyst to engineer selectivity from the outset can save a lot of time and effort purifying the product later on.
The challenge with this is that it is very difficult to change a specific site on a surface without also changing the environment around it. Now, researchers based in China have proposed a simple strategy to edit specific sites on gold nanoparticle catalysts without affecting adjacent atoms. In so doing, the team, led by Jing Ma and Yan Zhu of Nanjing University, achieved an increased degree of control over the regioselectivity of click reactions.
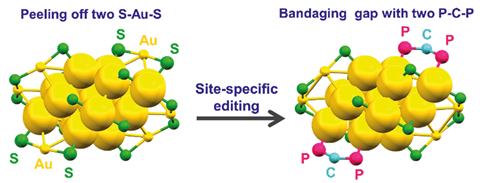
Their strategy involves preparing atomically precise gold nanoparticles protected by bulky phosphine ligands. Steric hindrance created by the ligands restricts how the substrates can approach the catalyst and therefore limits the number of options they have for how they react.
The specific reaction investigated in this study is the click reaction of benzyl azide and phenylacetylene, which can result in either a 1,4- or 1,5-triazole product. The gold nanoclusters as initially prepared, with thiolate ligands, do not demonstrate any selectivity for either of these products. A simple ligand exchange process to replace the thiolate ligands with larger phosphines introduces a bias towards the 1,4- product. This bias increases with the proportion of thiolates that are replaced with phosphines, which means the ratio of product can be selected by selecting the ratio of ligands on the catalyst. ‘Cycloaddition by means of click chemistry has been widely applied to generate high-value organic compounds,’ comments Zhu. ‘Both 1,4- or 1,5-triazoles are valuable in click chemistry, but higher selectivity towards one pure product is more useful.’
This level of control is the ‘holy grail of catalysis’ according to José Antonio Odriozola, an expert in catalysis based at the University of Seville in Spain. However, he says the researchers have not described why this strategy works as well as it does or which species catalyses the reaction. ‘They have evidence that something changes, but they don’t know exactly why. The real catalyst doesn’t change during the reaction, but we may have a precursor when we do the characterisation.’ Similarly, he wants to know more about the role of the ligands themselves. Odriozola explains that the active component in many metal nanoparticle catalysts is a layer of carbon that coats the surface and that the efficacy drops away if the surface is cleaned to make the particles pristine. Zhu acknowledges that there are still unknowns: ‘It is not easy to determine now how the modifications we make to the nanoparticle lead to more control.’ Odriozola suggests that investigating the kinetics of the process would be a good place to start building a clearer picture of what is happening.









No comments yet