Thionyl tetrafluoride-based ‘sleeping beauties’ enable click chemistry in a tetrahedral shape
Nobel laureate Barry Sharpless and his collaborators have smashed together two storybook-nicknamed scientific worlds, calling on ‘sleeping beauty’ chemical groups to break his famous click chemistry out of ‘flatland’.
The original click chemistry – clean, copper-catalysed triazole formation developed by Sharpless’ team at Scripps Research Institute in San Diego, US – has many uses today, including tagging biomolecules. However, the chemical groups it can be used with are largely limited to azides and alkynes, while the flat triazole molecule means attachments always form along a straight line.
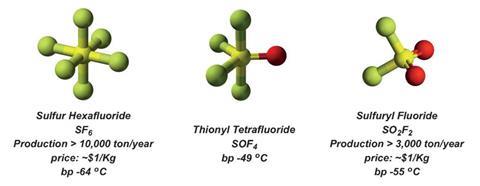
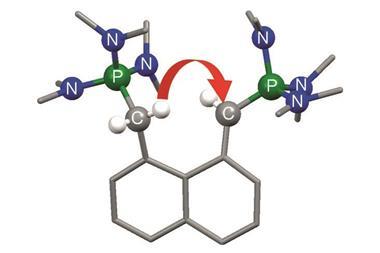
But now, click chemistry’s repertoire has been expanded significantly. The new approach starts from thionyl tetrafluoride (O=SF4) gas, onto which the scientists click functional groups from the important amine class, and phenols, over three steps.
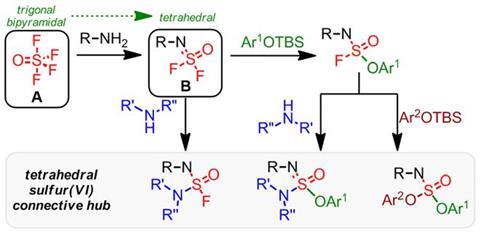
‘We demonstrate that three sequential reactions, each creating a unique connection, can be achieved with almost perfection,’ comments team member John Moses, from the University of Nottingham, UK. ‘These connections depart from a single sulfur hub into the 3D world, which is a first for a click connector.’
A primary amine can displace two of thionyl tetrafluoride’s four fluorine atoms to make a tetrahedral connective hub when a tertiary amine catalyst is present. Secondary amines, amino acids and protected phenols can displace a third fluorine atom, when an amine or related basic catalyst is present. The final fluorine from the product of reactions with protected phenols can be displaced by a secondary amine directly, or another protected phenol in the presence of a phosphazene base catalyst.
This temporary inertness, and subsequent controllable clean reaction, is exactly what constitutes click chemistry. According to Moses, the sulfur (VI) fluoride compounds’ stability until exposed to tertiary amines led Sharpless to dub them ‘sleeping beauties’.
‘This is a nice continuation of work from the Sharpless lab on extensions of click chemistry, creating more versatile methods with controllable three-dimensional control at the conjugating agent,’ comments Eric Anslyn at the University of Texas, Austin. He adds that it should help in fragment-based drug discovery, due to the simple assembly around a single core.
One limitation is that thionyl tetrafluoride is not currently commercially available, but the team is working with a Chinese company to develop a ton-scale process, Moses reveals. They are also biologically screening the sulfur-derived compounds they have produced and making new types of functional polymers. ‘This is a very exciting time for click chemistry,’ Moses says.
References
S Li et al, Angew. Chem. Int. Ed., 2017, DOI: 10.1002/anie.201611048











No comments yet