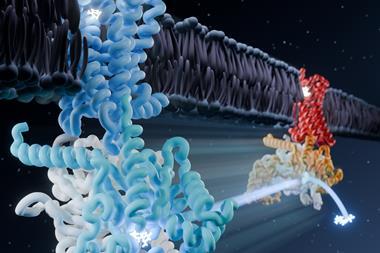
A series of highly detailed images of the structures found in the whirling flagellum that powers a bacterium’s movement has revealed new insights about how microbes get to where they want to be.
Using cryo-electron microscopy (cryo-EM) two different groups have uncovered the molecular architecture of, and interactions between, key components of the bacterial motor that powers bidirectional rotation of the flagellum.
‘The whole concept of chemotaxis is the ability to move away from danger and towards food sources and many bacteria are able to do this via the flagellum … either by changing the direction in which the flagellum rotates or by stopping it completely,’ explains Steven Johnson, staff scientist at the National Cancer Institute (NCI) in the US and first author on one of the studies.
Both Johnson’s team, who published in March, and the team led by senior research associate, Prashant Singh at Vanderbilt University in the US, who published in April, turned to the flagellum of the ‘workhorse’ bacterium Salmonella which also shares many similarities with Escherichia coli.
My grandfather built motors that powered big machines. In a lab not so different from his workshop, my colleagues and I uncovered the assembly of the bacterial motor. Today, this discovery has been published in @NatureMicrobiol #MolecularNodes #Discovery https://t.co/m98Df1DuOr pic.twitter.com/HgY9fsu1kC
— Prash (@prash_singh) April 17, 2024
‘[Salmonella] has a very simple system of turn counterclockwise to go in a straight line, turn clockwise to just start tumbling on the spot, and by combining those two different modes of operation, you can bias the direction in which the bacteria is walking,’ Johnson explains.
The motor in the flagellum is composed of multiple rings, one of which is the cytoplasmic ring (C-ring), or ‘switch’, which is responsible for switching the rotation of the flagellum between counterclockwise and clockwise.
Both research groups wanted to uncover the exact molecular mechanisms behind this rotation reversal of the flagellum.
‘It has been known for decades that the rotation direction did change and … that it’s this giant [C-]ring structure in the cytoplasm of the cell that’s responsible for that change and … that there’s a whole host of signalling receptors that respond to the external signals that then signal for this one particular molecule which combines to the C-ring motor and changes the direction the rotation occurs,’ Johnson says. ‘We also know that this rotation was partly being driven by small external stators … we learned a couple of years ago that that’s likely due to a mechanism by which the stators themselves have tiny little rotary motors…. This led to the hypothesis that the tiny rotary motors on the outside are driving this giant cog on the inside.’
However, Johnson says what they didn’t know was how to alter the C-ring and change the direction it rotates relative to these tiny external motors, because they’re rotating in one direction.
Changing direction
Johnson’s group worked on the full native assembly purified from the bacteria meaning they were able to design a construct fusing a piece of the cytoplasmic ‘cog’ onto the stator to allow them to get a high-resolution view of that specific interaction. They looked at both the clockwise and the counterclockwise rotating forms and were able to define a huge structural conformational change in the C-ring, enabling directional switching.1
‘This essentially means that these little rotating motors move from being on the outside [of the big cog] to the inside, so the direction in which they drive it is reversed because essentially there is a 180° swap in conformation,’ explains Susan Lea, chief of the Center for Structural Biology at the NCI, who led the team of researchers. ‘It’s this humungous change which really extends our understanding of how big a conformational change proteins are able to make,’ she adds.
The other team, led by Tina Iverson, Louise B McGavock Professor and a pharmacologist at Vanderbilt School of Medicine, US, reported cryoEM structures of three states of the flagellar switch, answering key questions about how the motor transmits torque and direction to the flagellum and observing the same 180° shift in conformation.2
However, Iverson says the questions they were trying to answer evolved over the course of the study. ‘The first questions that we had seem so trivial now – which is that this motor is powered by a gradient across the membrane of protons – a proton flux. It’s a current that runs down, transverses the membrane and can turn the rotor one way or the other. In this case, the current that goes one way can turn the motor either way and so we asked, how do you have something that’s reversible? That really wasn’t understood.’
‘What we see after this study is almost a trivial explanation, which is you have this giant motor and a piece of the motor that responds to the current just flips around to reverse the motor.’
Morgan Beeby, a structural biologist at Imperial College London, in the UK, welcomed the findings of the two studies. ‘Both papers determine the actual molecular architecture of a part of the motor we call the rotor – the cytoplasmic ring … the rotor component is interesting because it can also switch from rotating clockwise to counterclockwise and that’s the basis for how bacteria navigate… The key insight is a very clear, molecular rationale for how the rotor rings switches between a clockwise and counterclockwise conformation.’
‘In isolation this result may not be that exciting, but because we’ve now got the structure of the stator component as well … we can now start saying something quite intelligent about how the thing actually works.’
Beeby said it was possible to imagine that this research could be used as the basis for developing a therapeutic that blocks the ability of bacteria to navigate. ‘You might imagine inventing a small molecule that somehow sort of throws a spanner in the works of the switching of the C-ring from clockwise to counterclockwise conformations, and that would then abolish the ability of the pathogen to swim to a beneficial site,’ he says.
References
1 S Johnson et al, Nat. Microbiol., 2024, DOI: 10.1038/s41564-024-01630-z
2 PK Singh et al, Nat. Microbiol., 2024, DOI: 10.1038/s41564-024-01674-1























No comments yet