On the west bank of China’s Pearl River estuary, in Zhuhai, researchers are using renewable energy and seawater to shrink the petrochemical industry’s carbon footprint, via ‘green’ hydrogen. Scientists from Beijing University of Chemical Technology (BUCT) and the China National Offshore Oil Corporation (CNOOC) are building a pilot plant to directly split seawater into oxygen and hydrogen.
This is a massive scientific challenge because seawater can be corrosive and chemically complex. Meanwhile, wind and photovoltaic power sources run intermittently, explains BUCT’s Xiaoming Sun. In a Nature paper published in March, Sun’s team demonstrated a catalyst capable of resisting the ocean’s corrosive power and working as an electrode in a seawater electrolysis cell. This is especially important because during intermittent operation chemical reactions occur that could make the electrode less effective.
Why bother? Because, in a decarbonised world, green electricity and green hydrogen will be the primary energy sources, according to Julian Gregory from the University of Exeter. But renewable energy’s variability means the world must build excess capacity to ensure supply. This leads to surplus electricity when weather conditions are favourable. Since large-scale electricity storage is costly, it’s better to use excess energy to produce green hydrogen through electrolysis, Gregory says.
Is direct seawater electrolysis ‘a solution looking for a problem?’
The many daunting scientific and economic issues facing anyone seeking to make green hydrogen straight from seawater include competition from electrolysis following desalination. That alternative technology leads some to ask whether direct seawater electrolysis is ‘a solution looking for a problem?’ Yet researchers are convinced it has potential, leading to deployments like the Zhuhai pilot plant and a ‘factory-scale research initiative’ in Qingdao, China.

With the world’s carbon emissions continuing to grow, in defiance of international climate treaties, such technology is under pressure to deliver. Researchers need to find ways to solve intricate problems quickly and usher direct seawater electrolysis into niches profitable enough to prosper in. Perhaps paradoxically, reducing emissions in the oil and chemical industry could be one such niche.
In the UK, a University of Glasgow team may have solved some problems, as illustrated by an experiment involving Evian mineral water. We can taste that Evian and seawater have very different chemical compositions. Yet when it comes to splitting the water, in one way they’re surprisingly similar.
Evian knows
Electrolysis splits water into oxygen and hydrogen, in cells where electrons leave through an anode and re-enter through a cathode. Yet tearing apart such a stable molecule as water is difficult, requiring electrodes that can catalyse reactions to minimise how much energy is needed. They’re therefore often called electrocatalysts.
If you could feed seawater into electrolysers that would be a game changer
Mark Symes, University of Glasgow
Mark Symes’ Glasgow team tested one of the most common types of water electrolysis system. Experts expect proton-exchange membrane (PEM) cells ‘to be the most compatible with renewables’, says Symes. ‘Everyone says, “this is how we’re going to produce hydrogen”.’
The other dominant water electrolysis technology is based on alkaline cells that use a ceramic separator that allows hydroxide ions to pass through it, rather than a membrane. However, they are less well-suited to intermittent operation, says Titi Oliyide, an energy consultant with experience in water electrolysis. ‘Ramp up or ramp down, PEM can do that faster than alkaline,’ she says. PEM cells can also be more compact than alkaline cells, Oliyide says.
PEM cells let positively charged hydrogen ions, also known as protons, move from anode to cathode through a solid polymer membrane to balance the electron flow. The membranes have negatively charged functional groups that reject negatively charged ions. When the protons reach the cathode, the electrons reduce them to make hydrogen gas – in theory.
In practice, even the relatively small number of other ions found in Evian are enough to cause a PEM cell the Glasgow team had built to break down ‘within seconds’, Symes says. Sodium ions enter the membrane, but the cell doesn’t reduce them, meaning they block current flow, he explains, stopping the electrolysis process.
The sensitivity of PEM membranes is important, Symes explains, because we can’t make the amount of green hydrogen we need without seawater. And as we can taste, seawater is more concentrated than Evian, making its ability to cause breakdown ‘extreme’, Symes adds.
Desalinating seawater before electrolysis only adds a few percent to the final cost. But getting water pure enough to use in a PEM cell is ‘going to push the price up considerably’, Symes says. ‘If you could feed seawater into electrolysers that would be a game changer.’ Yet seawater electrolysis systems also face more common issues.
Developing solutions
Even without electrolysis, seawater corrodes metals by reduction, the metals losing electrons to water or dissolved oxygen, oxidising them. Meanwhile, on metal surfaces and in cracks, hydrogen can react and progressively weaken the material, known as embrittlement. ‘Hydrogen is not kind to metals,’ says Oliyide.
During electrolysis, seawater can change from acidic to alkaline and back again. At cathodes, where hydrogen gas forms, in alkaline conditions hydroxide ions can create precipitates with magnesium and calcium metal ions in seawater. Other trace metals can also deposit on the cathode alongside these precipitates. This makes the water-splitting reaction harder. At anodes, reactions producing chlorine from chloride ions can compete with the reaction producing oxygen.
Symes’ team’s cell can use a membrane that allows sodium through, along with a simple cathode based on carbon. At the carbon cathode, the cell captures protons and electrons using soluble clusters called polyoxometalates, such as silicotungstic acid. Such a system can pump the clusters to a separate area, where a catalyst such as platinum releases hydrogen. ‘The sodium ion can go into the solution that has the redox mediator in it, the membrane is not blocked,’ Symes says. ‘There’s no reason why the current should stop flowing.’ Symes likens the way the polyoxometalate solution carries hydrogen to how blood carries oxygen.
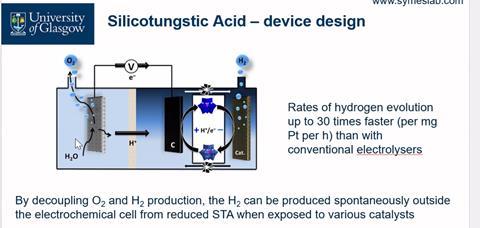
A spin-out company called Clyde Hydrogen Systems is now trying to commercialise the technology, with Symes as chief scientific officer. ‘Their first demonstrator will be about a kilogram of hydrogen per day,’ he says. ‘The idea is to scale up to 10kg, and then bigger.’ Corrosion will still happen, however, Symes concedes. ‘We need to make the system modular, so that you can swap bits in and out easily.’
Membranes are the most expensive and most vulnerable parts of current technology
Carl Fischer, Shyp
Membranes are also made of perfluoro- and polyfluoroalkyl substances (PFAS), known as ‘forever chemicals’ because they don’t degrade quickly, Oliyide adds. This is useful because they are stable in electrochemical environments. But ‘from an environmental perspective, that’s not great, especially when thinking about disposal’, she says. Some groups are therefore seeking to resolve membrane-related issues by developing electrolysis cells without membranes.
One company developing membraneless seawater electrolysis technology is Shyp, headquartered in Baltimore, US, with a lab in Aberdeen, UK. ‘Membranes are the most expensive and most vulnerable parts of current technology,’ says Carl Fischer, Shyp’s chief executive officer. ‘The salinity in the air alone is detrimental to membranes.’ While details of its approach remain secret, it will produce hydroxide chemicals as a byproduct, which provides revenue to reduce the cost of the green hydrogen it produces. The company currently has a benchtop pilot system and has been awarded an EU grant to install a full pilot system in Rotterdam by 2028, Fischer says.
Sun’s BUCT team also avoids PEM cells, instead opting for an anion-exchange membrane (AEM) system. AEMs don’t need substances to move through solutions separated by a membrane. Instead, a polymeric electrolyte can separate the electrodes, with water entering and hydrogen leaving on one side, and the cell generating oxygen on the cell’s opposite side. However, such systems are not yet widely used commercially because hydroxide ions degrade the polymers.
Entering competition
The anode and cathode electrocatalysts in water electrolysis cells are often metallic foams made of nickel and iron alloys. To boost their catalytic activity and stability, researchers explore alloys containing up to five elements. Scientists can also alter electrocatalysts’ structures, for example coating them with protective shells. In their March Nature paper, Sun’s team developed a stable NiCoP–Cr2O3 electrocatalyst that resists corrosion.
Simulating frequent start–shutdown seawater electrolysis, they discovered halide ions from seawater adsorbing onto the cathode, and currents oxidising it, reducing hydrogen output. Their catalyst forms a phosphate-based layer that protects it from these processes. It then successfully reactivates when hydrogen evolution restarts. And while the knowledge it provides will no doubt help the Zhuhai pilot project, direct seawater electrolysis must catch up with technology that desalinates the water first.
The world’s largest green hydrogen production plant, in construction in NEOM, Saudi Arabia, plans to use 4GW of solar and wind energy. It is due to produce up to 600 tonnes per day of carbon-free hydrogen by the end of 2026 from desalinated seawater. At 1MW power consumption, the BUCT/CNOOC pilot in Zhuhai is around one-four thousandth the size of NEOM. Yet CNOOC may opt for seawater electrolysis as an option better suited to floating platforms, providing a green energy source when renewables are not operating. Offshore platforms wouldn’t have enough space for desalination equipment, Sun notes. And even in remote desert locations like NEOM, direct seawater electrolysis may have an advantage in reducing the number of systems needing maintenance.
The Sinopec Qingdao plant is about one-37,500th the size of NEOM, at around 16kg per day. It harnesses green electricity generated by Sinopec’s nearby solar power station. Sinopec says that it has tackled corrosion using ‘specially designed key equipment and unique processes’. It intends to use the green hydrogen produced in its oil refining operations, potentially cracking large molecules to make smaller ones, and removing impurities like sulfur and nitrogen, and as a fuel source for hydrogen-powered vehicles.
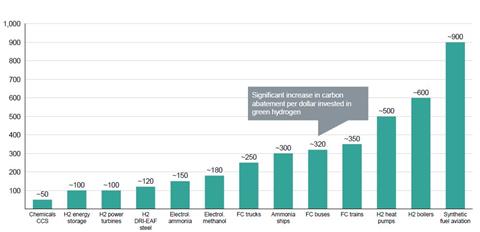
But Gernot Wagner from Columbia Business School says that using hydrogen in small vehicles ‘doesn’t make sense’. ‘Electric engines are simply significantly more effective at converting energy into distance,’ he says. He says that carmakers are still developing hydrogen-powered models due to a ‘mistaken belief’ that consumers would prefer them. He likewise adds that electric-powered heat pumps are superior to hydrogen-powered heating. Wagner points to research from Goldman Sachs showing that chemical applications, when combined with carbon capture and storage, are where green hydrogen most economically abates carbon emissions.
Seawater electrolysis technology relies on the success of green hydrogen but must establish itself in niches where space is limited or maintenance is difficult to enter industry. At the same time, green hydrogen is seeking to establish itself in niches like oil and chemical production. By displacing fossil-fuel-derived hydrogen, known as grey hydrogen, in those applications it can then expand to help decarbonise energy on a larger scale.
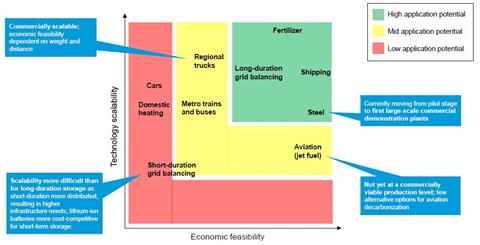
‘There’s a good chance electrolysers will someday be so cheap as to make green hydrogen competitive with grey hydrogen, but that takes massive investments,’ says Wagner. ‘We’re beginning to see investments, with LONGi, one of the world’s largest solar producers, now moving into electrolysers. But it’s still early days.’

















No comments yet