(‒)-Pavidolide B
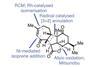
An innovative approach to making five-membered carbon rings makes for a strikingly short synthesis
There is no shortage of good ways to make 6-membered rings. Perhaps the most general and powerful is the venerable Diels–Alder [4+2] cycloaddition, an amazingly useful reaction that’s simple enough to teach to undergraduates, yet still a common sight in the primary literature. Unfortunately, tackling five-membered rings using cycloaddition chemistry is far less straightforward. While [3+2] and [4+1] cycloadditions are clearly mathematically feasible, in reality these reactions are tricky for all but a few special cases, and are not a common approach to all-carbon rings. That’s a shame, because five-membered carbocycles are a common feature in natural products and other interesting molecules.