A technique for forming new carbon–carbon bonds at specific sites in proteins
In the effort to find new methods to diagnose and cure diseases, scientists have turned their attention to post-translational modifications of proteins – adding functional groups or other proteins to a protein freshly produced from its genetic instructions. Post-translational modifications alter the properties of the original proteins, which can greatly expand their structures and functions, and regulate cell activity and reaction sites. For example, adding one or more units of the protein ubiquitin to other proteins plays an important role in regulating the occurrence, development and metastasis of several cancers.
In nature, the cell’s protein modification process has been refined by millions of years of evolution, but only works for natural amino acids. Chemical techniques to achieve specific modifications without restrictions face certain challenges. Firstly, the reaction needs to be carried out in the water phase, since the protein is often only soluble in water. Secondly, the chemical reaction conditions must be mild enough to avoid protein inactivation. Thirdly, the chemical reactions need to be reasonably selective, because proteins often have many functional groups with different properties. Therefore, the application of site-specific biomolecule modification is more restricted, and at this early stage of development is mainly limited to peptides, not whole proteins.
In 2016, Benjamin Davis from the University of Oxford, UK, brought free radicals into the field of protein modification, and reported the first general method for generating a new carbon–carbon bond on the dehydroalanine site of a protein to add both natural and unnatural side chains (figure 1).1 Dehydroalanine is a highly active free radical receptor that contains polarised double bonds. It also exhibits significant synthetic flexibility and can be easily installed in peptide and protein sites that need to be modified to participate in the reaction with the side chain. This differs from previous methods used to introduce post-translational modifications to proteins, which formed new bonds with heteroatoms in the side chain through processes such as phosphorylation, glycosylation and disulfide link formation. Even bioorthogonal chemistry can only form carbon-heteroatom bonds, not carbon–carbon bonds.
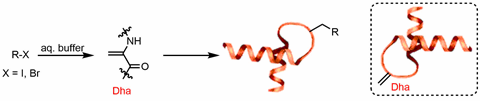
Since then, scientists have developed free radical reactions suitable for modifying whole proteins. This has been a difficult task because proteins contain many functional groups with redox activity, so the reaction conditions must be mild enough to selectively react with the target free radical precursor instead of the protein side chain.
In 2020, Davis and Veronique Gouverneur, also from the University of Oxford, led teams that collaborated on a new method of visible light induced protein functionalisation.2 They used catechol borate (BACED) derivatives and pyridine difluoroalkyl sulfone (PySOOF) as two complementary free radical precursors to functionalise proteins containing dehydroalanine in the water phase (figure 2). BACEDs are generated in situ as reducing free radical precursors to produce RH2C·, which can then form a link between the protein and the post-translational modification. PySOOF, as an oxidising free radical, produces RF2C· under the action of Fe(ii) to obtain a modified linkage that contains a CF2 label. This provides a powerful method for studying protein activity by 19F NMR.
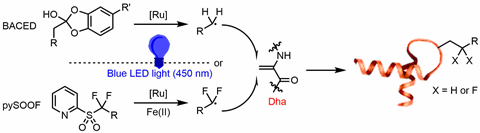
The two free radical precursors are easily obtained and modified by controlling the oxidation–reduction properties of the reaction. A high conversion rate can be achieved, and damage to the protein can be reduced, as well as providing a convenience for adding functional groups to the protein under neutral redox conditions. This strategy works with more than 50 types of residue and side chain modifications.
The modified proteins have good biological activity. Moreover, using catechol borates to add carbon–halogen bond active sites to the target protein allows cross-linking to adjacent proteins. Complex protein inhibition can be achieved by modifying the cross-linking of active site residues, with an effect similar to that of emerging targeted small molecule covalent inhibitors.
Protein post-translational modification is currently a hot research field, and new methods and technologies are constantly being developed. Although they are not perfect yet, they still have huge potential for application. The site-specific nature of the modifications may help to develop protein drugs and covalent inhibitors in the future, forming a link between modern organic chemistry and the life sciences.
References
1 B G Davis et al, Science, 2016, 354, 6312 (DOI: 10.1126/science.aag1465)
2 V Gouverneur et al, Nature, 2020, 585, 530 (DOI: 10.1038/s41586-020-2733-7)











No comments yet