A complex synthesis is always more impressive when conducted on gram scale
Why do we do total synthesis? Regular readers of this column will have seen plenty of the exciting new methods that chemists can discover when forced to innovate to reach a new target. And even in this age of gigahertz NMR, benchtop density functional theory calculations and high resolution x-ray crystallography, total synthesis plays a role in structure elucidation. More than anything, I hope I’ve been able to provide a glimpse of nature’s molecular beauty, and how total synthesis is an edifying intellectual (and even artistic) pursuit. Somewhat more prosaically, though, sometimes we simply can’t get enough of interesting materials from nature itself.
For a potent natural product, a few grams can be enough to fill the in any gaps in its biological data and start to probe its potential as a therapeutic lead. A few memorable examples of gram-scale total syntheses of promising complex natural products such as spongistatin1 and discodermolide2 have come out of academic labs, but such efforts are extremely rare. At the end of my PhD I remember being immensely proud to have prepared almost 100mg of a simple natural product. It’s much more common to see a long total synthesis culminate in a fraction of that amount.
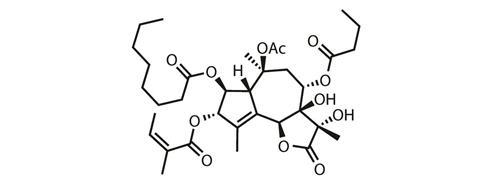
Phil Baran and coworkers at the Scripps Research Institute in La Jolla, US, have added thapsigargin to that list.3 Their achievement is made even more impressive by the complexity of the target, and the sheer number of oxygen atoms it contains. Over half of the carbon atoms bear an oxygen substituent, a proportion almost unheard of among terpenes, and a feature that complicates synthesis at any scale.
The group begins with dihydrocarvone, a simple terpenoid building block made cheap by demand from the fragrance industry. This is condensed with ethyl vinyl ketone in a classic Robinson annulation (figure 1). But the group adds a clever twist, bubbling oxygen through the crude reaction mixture to accomplish a diastereoselective γ-hydroxylation of the enone in the same step. Amazingly, this simple union of two commodity chemicals completes the group’s biomimetic ‘cyclase phase’ – introducing every single carbon atom found in the molecule’s core, albeit with the rings being not quite the right size.
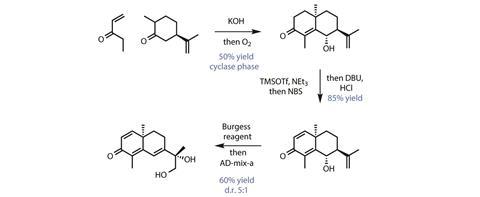
The ‘oxidase phase’ begins with a one-pot silyl enol ether formation-bromination-elimination sequence that oxidises the enone to the dienone. A little hydrochloric acid then desilylates the hydroxyl group, which is dehydrated with a dose of Burgess reagent to give the tetraene. Finally, a striking regio- and diastereoselective Sharpless asymmetric dihydroxylation oxygenates the terminal olefin on the side chain.
The team’s most remarkable reaction – and the reason for working with two seemingly wrong-sized rings, comes a few steps later. It’s a twist on some vintage 1950s photochemistry from British Nobel laureate Sir Derek Barton. Irradiating a solution of the 6,6-bicycle in acetic acid with a mercury lamp, triggers an unusual isomerisation to the 7,5-guaianolide ring system (figure 2). At the same time, an acetoxy group is diastereoselectively installed at C10 – exactly where it’s needed for the natural product. The catch is that the reaction must be very dilute, which somewhat dents its scalability. If thapsigargin turns out to be commercially valuable, this is one step the process chemists will need to work on.
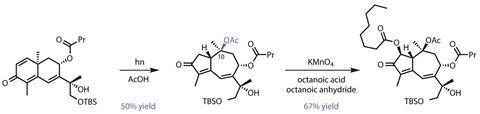
Another unusual oxidation follows, using potassium permanganate to perform an α-octanoylation of the cyclopentenone carbonyl. This is impressive as although potassium permanganate is cheap and much less toxic than chromium, osmium and selenium alternatives, it can be quite unselective and is rarely kind to complex molecules with this many oxidisable groups.
From here, most of the heavy lifting has been done, and just a few more oxidations complete the target. True to their aim, the researchers report every step on near gram-scale (except the last two, due to the extreme toxicity of the target natural product). This is a remarkable total synthesis of a complex natural product that would have been impressive on any scale!
Our columnist Mark Peplow considers this synthesis in his article asking what constitutes a step in organic synthesis?
References
1 A B Smith III et al, Org. Lett., 2008, 10, 4539 (DOI: 10.1021/ol801792k)
2 A B Smith III et al, J. Am. Chem. Soc., 2000, 122, 8654 (DOI: 10.1021/ja0015287)
3 H Chu et al, ACS Cent. Sci., 2016 ((DOI: 10.1021/acscentsci.6b00313)











No comments yet