Ben Valsler
A bright pink antiseptic solution certainly stands out from the crowd and, as Mike Freemantle discovers, the compound at the core of Hibiscrub and similar colourful cleansers has an important role in modern surgery and a history linked to one of the world’s biggest killers.
Michael Freemantle
Earlier this year, I spent a day in our local hospital. I was booked in for a surgical procedure to be carried out on my chest under local anaesthetic. Before the surgeon made the incision, a nurse thoroughly cleaned my chest with an antiseptic solution. I asked her what the antiseptic was. Chlorhexidine, she replied, adding that they used to use an iodine solution.
I was familiar with chlorhexidine. My dentist had strongly recommended that I regularly brush my teeth with a dental gel containing the compound to help prevent and treat gum disease.
Chlorhexidine is a type of organic compound known as a biguanide. It is used as an antimicrobial ingredient in skin disinfectants, creams and wipes, wound dressings, deodorants, toothpastes and numerous other products.
Its development as an antiseptic and disinfectant has a long history. During the Second World War, two British chemists, Francis Curd and Frank Rose, carried out extensive work at ICI’s research laboratories in Manchester on the syntheses of antimalarial drugs. In November 1945, they announced the discovery of a new drug, a biguanide now known as chlorguanide or proguanil. It is still used today to treat and prevent malaria.
Curd’s life and career were tragically cut short in December 1948 when he died from injuries sustained a few days earlier in a railway accident at Stockport, Cheshire. He was 39 years old.
Rose and colleagues at ICI, however, continued to investigate biguanides, or ‘diguanides’ as they called them. In 1954, the team reported that one class of substituted biguanides ‘exhibited marked antibacterial activity in vitro against a wide range of micro-organisms’. They compared the activities of 14 of these derivatives and showed that one ‘had the most outstanding … properties’ against bacteria such as Staphylococcus aureus. The compound, which was given the ICI trade name Hibitane, is a white crystalline, strongly basic solid that is only slightly soluble in water. The team therefore used the water-soluble diacetate salt of the compound for their studies.
Two years later, Rose and his ICI co-researcher Geoffrey Swain described the preparation of water-soluble dihydrochloride salts of Hibitane and related biguanides in a paper published in the Journal of the Chemical Society. They noted that Hibitane had ‘recently been introduced into medical and veterinary practice under the common name chlorhexidine.’
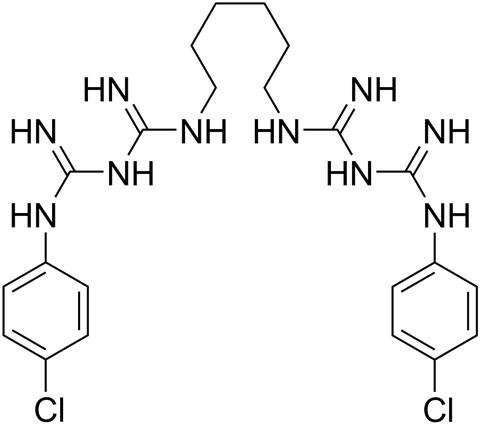
Nowadays, the digluconate salt of chlorhexidine is commonly used in biocide products. The chlorhexidine cation has two biguanide moieties, each of which contains five nitrogen atoms and is attached to a chlorophenyl group. The compound is therefore sometimes known as a bisbiguanide. Rose and colleagues called it a bisdiguanide. Confusingly, chlorhexidine digluconate is also referred to as chlorhexidine gluconate. The gluconate anion, and there are two of them for every chlorhexidine cation in the salt, is a derivative of gluconic acid. The acid is an open-chain form of glucose that occurs naturally in fruit, honey and wine. It is produced commercially by fermenting certain types of fungi.
Chlorhexidine digluconate and its other salts are cationic antiseptics. The positively charged chlorhexidine cations bind to the negatively charged cell walls of bacteria. The process disrupts the cell membranes allowing the cell contents to spill out. The activity of the salts against bacteria depends on their concentrations. Low concentrations prevent bacterial reproduction whereas higher concentrations kill the bacteria outright.
Every antiseptic has its pros and cons. Chlorhexidine salts have a broad-spectrum of biocidal activity. They exhibit excellent residual activity after being applied to the skin compared to iodine-based antiseptics which have lower residual activity. One recent report indicates that after a two-minute application to the skin, chlorhexidine antiseptics continue to kill bacteria for 24 hours. They are also fast acting, although not as fast as the ethanol and iso-propanol that are currently employed in the ubiquitous alcohol-based hand sanitisers.
Chlorhexidine salts also exhibit good activity against enveloped viruses. The envelopes are outer coatings that protect viral genetic material from the host immune system. Coronaviruses, such as the one that causes Covid-19, are enveloped viruses. So, to what extent is chlorhexidine used in commercially available hand sanitisers to help prevent the spread of the disease?

On a recent shopping expedition to our town centre, I couldn’t find one over the counter hand sanitiser with chlorhexidine in its list of ingredients. A few, however, were available for purchase online.
There is universal agreement that thoroughly washing your hands with soap and water is the best option for hand hygiene. If access to soap and water is not available, for example at the entrances of shops, then almost any commercial hand sanitiser – even if it only contains alcohol and water – is the next best option.
Ben Valsler
That was Mike Freemantle with chlorhexidine. Next week, Katrina Krämer finds out why a class of solvents may be set to change the world.
Katrina Krämer
I can say from experience that searching for the perfect solvent is not something many chemists enjoy. So I was surprised to discover that a class of solvents was once voted ‘the British innovation most likely to shape the 21st century’, sharing the honour with things like the Higgs’ boson, 3D-printed organs and the Raspberry Pi computer.
Ben Valsler
But what class of solvents could have such an impact? Find out more with Katrina next time. Until then, find all of the compounds we’ve covered at chemistryworld.com/podcasts, and let us know if you think we’ve missed something important – email chemistryworld@rsc.org or tweet @chemistryworld. I’m Ben Valsler, thanks for joining me.
Additional information
Theme: Opifex by Isaac Joel, via Soundstripe













No comments yet