Meera Senthilingam
This week, we begin with a story paving the way for the chemistry of a good compound keeping the bad ones at bay. Telling this tale is Phil Robinson
Phil Robinson
In a 1950s issue of Boy's Life magazine, amid the camping guides, fishing tips and air-rifle adverts, sits a story that contains some very contemporaneous chemistry.
On a field trip with a local geology professor, our schoolboy protagonist, Scoops Traylor, uncovers a Native American idol, somewhat improbably made of pure uranium. Unbeknown to Scoops and the rest of the group, one of the boys can't resist pocketing a fragment and the light-fingered lad is later discovered in the throes of acute radiation poisoning.

Happily, Scoops - the precociously resourceful son of a physicist - is quick to recall how his father's lab deals with fissile fouling: 'At Dad's laboratory, the technicians use a solution called versene to wash radioactive contamination off their equipment.'
'There's some versene in my van,' responds our geologist, who turns out to have a handy sideline in uranium prospecting. The miscreant is thoroughly doused and flushed with versene and Scoops is hailed a hero.
The wide-eyed excitement of this story certainly contrasts with today's chemical-averse attitudes (although few will mourn the passing of its rather crude and objectionable portrayal of Native Americans). And compared to some of the more outlandish science fiction that appeared following science's mastery of the atom, it is a story very much rooted in science fact.
That solution - versene - is still available today, manufactured by Dow, and still sold as a cleaning agent. But today it is listed with a host of other applications too, for use in producing everything from food to textiles and pharmaceuticals. And the compound responsible for versene's versatility is ethylene diamine tetraacetic acid, or EDTA.
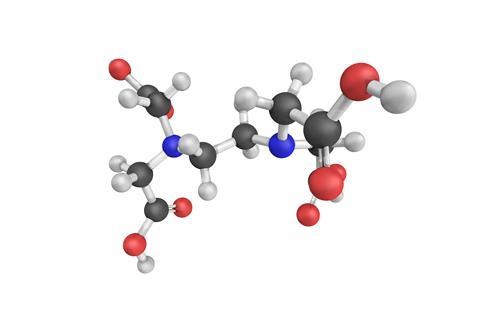
EDTA is a polyamino carboxylic acid. Two amine centres, sharing an ethylene bridge, form the molecule's core, and from each nitrogen atom, two carboxylic acid arms issue forth. This structure is reminiscent of the four-fingered claws found in fairground 'crane games'. And its chemistry is pretty similar too: in all of its uses, EDTA acts as a chelating agent, a term derived from the Greek word for claw. But rather than grabbing cuddly toys, the EDTA claw is expert at grabbing metal ions.
The term 'chelate' was originally coined in 1920 to describe interactions where a ligand molecule coordinates a metal ion through two of its atoms, in a pincer-like arrangement similar to the claws of a lobster, but now it applies generally to any ligand capable of multiple coordination. When EDTA is fully deprotonated, its two amino nitrogen atoms and four carboxylate groups provide six points with which to bind a metal ion, which enables it to wrap itself around the ion, enveloping it in an octahedral embrace. This multiplicity of binding gives EDTA a high affinity for a range of metal ions and it is this affinity that gives EDTA its superlative properties.
The molecule was first synthesised in the 1930s by Ferdinand Munz, then working for the chemical company IG Farben. To reduce the Nazi government's reliance on foreign chemical imports, Munz was working to find a replacement for citric acid. Citric acid (itself a chelating agent) was used as a water softener - removing calcium ions during textile dyeing to prevent discolouration. Munz noted that an amine polycarboxylic acid performed much better than citric acid (which is a polycarboxylic acid), and presumably reasoned that a polyamine polycarboxylic acid might be even better. He was right.
EDTA is still used as a water softener today, but that was just the beginning. EDTA's ability to sequester a range of metal ions makes it useful for removing metal ions wherever they are found. And they are found everywhere.

But it's not just about removal - as it grasps the metal, EDTA restricts the access of other species to the metal. Thus, catalytically active metals can be isolated and prevented from interfering with other reactions. This use is particularly important in the paper industry, where EDTA will prevent manganese ions catalysing the breakdown of hydrogen peroxide - the bleaching agent used to whiten paper.
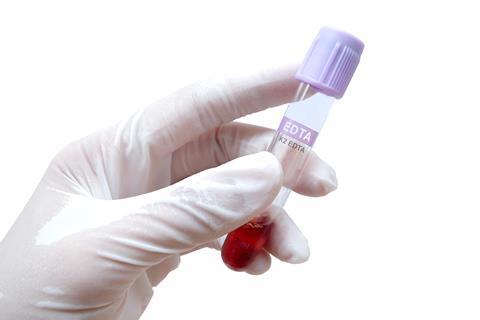
Similarly, EDTA acts as a preservative by removing metal cofactors required by enzymes that would otherwise cause food to spoil. This anti-enzymatic function also finds EDTA a use as an anticoagulant: by removing the calcium ions that are an essential part of the cascade of reactions leading to blood clotting, EDTA can be used to prevent the coagulation of blood samples and fouling of clinical or surgical equipment.
In analytical chemistry, EDTA is the compound of choice for standardising solutions of metal ions. What undergraduate lab course would be complete without the numbing repetition of endless volumetric titrations? Thank you, EDTA.
And around the time that Scoops Traylor's improvisation showed how something that cleans an instrument could also 'clean' a person, EDTA was receiving the attention of the medical community in the emerging field of chelation therapy. Chelation therapy uses chelating agents to remove poisonous metals from the body, and it began during the second world war when chemists at the University of Oxford developed a chelating agent to use as an antidote to the arsenic-based chemical weapon, lewisite. Other chelating agents were soon being investigated and EDTA was found to be particularly effective in the removal of lead.
After the second world war, EDTA was used to treat the workers who had painted US Navy vessels with lead-based paints, and it remains one of the first-choice treatments for acute lead poisoning. The toxicology of lead is now well known, so lead exposure levels are much less than they once were but cases of severe lead poisoning still occur. In 2010, villagers in the Nigerian state of Zamfara suffered a lead poisoning epidemic after mining for gold in areas heavily contaminated with lead. Sadly, hundreds died, most of them children, but the figure would have been much larger had it not been for the intervention of chelating agents such as EDTA.
Early reports from patients who received chelation therapy for metal poisoning suggested that EDTA also alleviated the symptoms of coronary artery disease. However, the consensus of the medical community today is that EDTA delivers no benefit in such instances and this treatment remains firmly outside the realm of conventional medicine. More controversially, EDTA is recommended by practitioners of complementary medicine as a treatment for autism and Alzheimer's, neither of which is supported by clinical studies.
As a metal-grabbing chemical claw, EDTA may be something of a one-trick molecule. But EDTA has certainly mastered that trick and made the most of it, making EDTA a very useful - and memorable - compound, from the lab to the emergency medical kit.
Meera Senthilingam
Chemistry World's Phil Robinson there with the chelating chemistry of EDTA. Next week, another versatile compound giving some companies a rather hefty profit from its chemistry.
Tim Harrison
Silicone chemistry is a ten billion dollar plus per year industry. Silicones are present in many familiar products and applications as diverse as shampoos and personal grooming products through bathroom sealants, water-proofing and environmentally-friendly dry cleaning agents. They also have numerous medical uses.
Meera Senthilingam
And discover the chemistry enabling all of these uses by joining Tim Harrison in next week's Chemistry in its element. Until then, thank you for listening, I'm Meera Senthilingam.













No comments yet