Meera Senthilingam
This week, the compound bringing some colour, and oxygen, into our bloodstream. Here's Brian Clegg:
Brian Clegg
If you play word association starting from 'blood', it won't be long before 'red' comes up. Blood's deep red hue is probably its most distinctive characteristic. Look closer at the red fluid that seeps from a wound under a microscope and you will see that the red colouration comes from tiny objects shaped like dried apricots, each a single cell that typically passes around your body every 20 seconds during its four month life span. And these red blood cells owe their colouration to the compound haemoglobin.
This is, in effect, a nickname for this essential protein. Until the 1860s it would have been referred to as haematoglobulin, a name that combines the Greek for blood, haima, and the idea of a globule - it's a little blood blob. Haemoglobin is not unique to humans - in fact every vertebrate apart from fish makes use of this tangled molecule as a vehicle to carry oxygen around the body and to transport back carbon dioxide to be expelled. It's the body's equivalent of a gas tanker.
Compared to most cells in a human body, red blood cells are very simple. They have no nucleus, nor any DNA. In fact remove the water, and over 95% of what's left is haemoglobin. These are cells with a very clear mission. Each haemoglobin molecule in a mammal can carry four molecules of oxygen around the body.
The compound was discovered in 1840 by Friedrich Ludwig Hünefeld, by pressing the blood of an earthworm between two glass slides and allowing it to dry and crystallise, while its role as a carrier of oxygen was suggested by the French physiologist Claude Bernard around 1870. Like many proteins, haemoglobin's structure was too complex to determine until x-ray crystallography made it possible. The details were determined by Max Perutz in 1959, shortly after the publication of the structure of DNA.

In human haemoglobin there are four major units, each containing a smaller, but highly significant motif called a 'heme' group. This group contains a single iron atom, held in the centre of a square described by four nitrogen atoms and exists at the heart of an array of organic rings called a porphyrin. It is this iron that binds oxygen and allows haemoglobin to do its job.
The iron in haemoglobin is often thought to account for the distinctive red colouration of our blood, in the same way that iron oxide gives rust its colour. As it happens, here the iron content is just a coincidence - the red colouration in fact comes from the porphyrin (the word 'porphyrin' derives from a Greek word for a reddish purple). Just how red the haemoglobin is, depends on whether there is oxygen bound to it. When there is oxygen present, it changes the shape of the porphyrin, making the colour a brighter red.
For that matter, not every red liquid that oozes from flesh is coloured by haemoglobin. The red hue of raw meat, and the thin red liquid that oozes from it, which we often call blood but isn't, is coloured by myoglobin, a simpler molecule with just a single iron atom that is used to store oxygen, rather than to transport it around the body.
Although haemoglobin does the job of carrying oxygen well, it is susceptible to some threats. Carbon monoxide, for instance, is a colourless, odourless gas that binds even better to the iron in haemoglobin than does oxygen. In fact it is preferred to such an extent that it only takes a relatively small amount of carbon monoxide in the atmosphere to block off enough of the haemoglobin to render someone unconscious and to cause asphyxiation - which is why carbon monoxide leaking from faulty appliances can be such a deadly danger.
Foetal haemoglobin is subtly different from the adult form, with a better ability to grab onto oxygen than the molecule present in adults. This is because the foetus gets its oxygen from the placenta, and by the time blood gets there, the mother's body will have taken much of the oxygen for itself. Foetal haemoglobin therefore has to be particularly good at getting hold of oxygen to compete with the mother's needs.
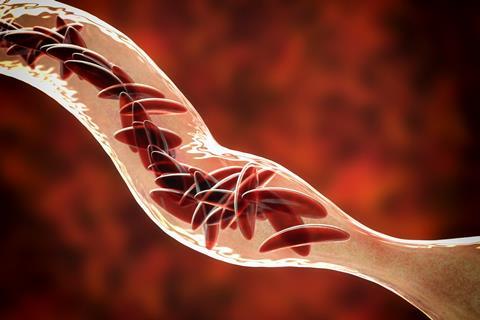
There are also different forms of haemoglobin that can cause illness. Sickle cell anaemia, for example, is caused by an abnormal haemoglobin structure, which reduces the oxygen carried in a red blood cell and alters its shape to the distinctive crescent moon form.
Haemoglobin, then, plays an essential role in our respiratory system, providing the transport for the oxygen that will be used in the slow burn of the chemical reactions releasing energy to power our muscles. It's the trucker of the bloodstream in its convoys of red blood cells, each molecule making up to three deliveries a minute to keep you and I moving on down the road.
Meera Senthilingam
So, in its many forms, we all have haemoglobin to thank for the energy that gets us through the day. That was Brian Clegg with the oxygenating chemistry of haemoglobin. Now, next week: prepare for some explosions.
Simon Cotton
When a compound is best known by an acronym, that usually means bad news. Just think of DDT, the insecticide, or GHB, which is best known as a 'date rape' drug. And then there is TNT, whose chemical name is 2,4,6-trinitrotoluene.
TNT had several advantages over other explosives. Although it is not as strong an explosive as picric acid, it was much safer to handle and harder to detonate. Because of its melting point of only 80?C, it became possible to fill shells safely with molten TNT. The German military started using TNT as their standard explosive in 1902, whilst the British stuck with picric acid, a choice with unfortunate consequences.
Meera Senthilingam
And you can find out the more fortunate consequences and advantages of using the compound TNT by joing Simon Cotton in next week's Chemistry in it's element. Until then, thank you for listening. I'm Meera Senthilingam.














No comments yet