Meera Senthilingam
This week, a deadly compound. Here's Akshat Rathi.
Akshat Rathi
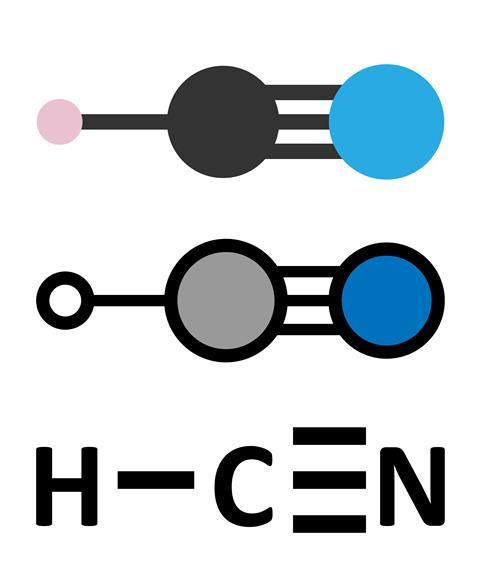
Hydrogen cyanide is a very small, linear molecule. It is simply H-C-N. The carbon atom is connected to the nitrogen by a strong triple bond, whereas the hydrogen is much more weakly attached. Plucking off the hydrogen atom leaves the negatively charged and highly reactive cyanide ion, CN-.
Cyanide is known to form strong bonds with metals, and this property is exploited in purifying gold from its ore. Gold ore is stirred in vats that contain a solution of sodium cyanide to form soluble gold cyanide complexes. Although very large amounts of this highly poisonous material are used, there is nothing to fear because almost all of it is recycled. Nevertheless, the technique remains controversial because of the risk of accidentally releasing large amounts of highly toxic cyanide compounds into the environment.
Cyanide is one of the quickest acting poisons, hence it is famously referred to many times in thriller stories, as a murder weapon or in the form of a suicide pill given to secret agents in case they are captured. Its toxicity arises from the affinity of the cyanide ion to iron atoms. When cyanide ions are present in the human body, they quickly bind to iron atoms inside cells. In doing so, they inhibit an important enzyme - cytochrome C oxidase, which occurs inside the energy-producing mitochondria of a cell. The enzyme is essential to life because it catalyses the final stage of glucose oxidation. When it is blocked, the source of energy within the body quickly dries up, immediately affecting the central nervous system and the heart. Binding of cyanide to the iron atom is irreversible in the sense that it can only be removed by chemical attack. On cyanide consumption, within minutes the victim becomes unconscious although continues breathing, and slowly the heart gives out, causing death.
Hydrogen cyanide's action as poison is not just restricted to the movies, though. Some varieties of Cassava, which is a staple food of 500 million people, contain enough cyanide to kill six people per kilogram of the crop. Fortunately though, the methods used to cook Cassava ensure that, if done properly, a person eating a kilogram of Cassava will receive only one-fifth the lethal dose of cyanide. The white roots must be soaked or boiled in water, or fermented to remove the cyanide. This processing is often done industrially to produce safe foodstuffs like cassava flour and tapioca. Despite the availability of techniques that allow for safe consumption, cyanide poisoning by Cassava became a common problem in the 1980s, especially in the drought afflicted areas of Africa where people were not willing to take the right measures to prepare Cassava before consuming it. Seeds of fruits like apples, cherries and almonds also contain HCN, but in such small amounts that they pose no health risks.

But as bad as cyanide poisoning seems, a number of antidotes have been developed and their mechanism of action enables the cyanide ion to latch on to another molecule instead of binding to the iron atom in cytochrome oxidase. One such sacrificial molecule which is available in the human body is haemoglobin. But to enable the iron atom in haemoglobin to pick up the cyanide ion, it is necessary to oxidise Fe(II) to Fe(III). This can be achieved by injecting either sodium nitrite or 4-dimethylaminophenol. Other sacrificial molecules that can be introduced into the human body are hydroxycobalamin, a relative of vitamin B12 - or kelocyanor, which both employ cobalt to mop up cyanide ions.
Bearing this in mind, it could come as a surprise that chemical industries around the world produce enough hydrogen cyanide every month to kill every living person on Earth. But of course, most of it is used to produce a wide variety of organic compounds.
For example, adiponitrile - made by adding HCN across the two double bonds of butadiene - is a precursor to the polymer Nylon, which is used for a variety of applications from making composite materials to being used as sutures after surgery. It is also used to synthesise the essential-to-life amino acids for commercial use.

The ability to easily form a plethora of organic compounds, with many reagents and under a variety of conditions, has made researchers think about hydrogen cyanide's role in the origin of life - leading to an ongoing debate. Those supporting the argument claim that hydrogen cyanide could have well been formed by lightning discharges in the prehistoric atmosphere of our planet. The presence of other chemicals at the time may have enabled the synthesis of the amino acids that form the basis of our life.
Whichever way the debate goes, hydrogen cyanide is a fascinating molecule - maybe the giver, sometimes the taker, and in many ways today also the supporter of life.
Meera Senthilingam
Indeed, but I still think I might take a bit more care when next using cassava as an ingredient in my meals. That was Akshat Rathi, with the toxic but useful chemistry of hydrogen cyanide. Now next week, a compound to stop your clothes getting old before their time.
Josh Howgego
One day I noticed that a pair of my jeans was developing a couple of small holes. Gradually I started noticing more and more items developing frays and splits.
Suddenly it hit me: I had a moth problem. I started to read all about silk moths in an effort to see if there wasn't some way in which I could rid myself of these loathsome Lepidoptera. Almost immediately, I was fascinated by the story of Bombykol, the sex pheromone of the female silk moth.
Meera Senthilingam
And to find out how this pheromone was first discovered, as well as its uses to keep pests at bay, join Josh Howgego in next week's Chemistry in its element. Until then, thank you for listening. I'm Meera Senthilingam















No comments yet