Walking protein’s tiny steps measured with germanium nanospheres
Highest ever resolution measurements suggests answer to kinesin dispute
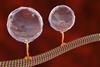
Researchers in Germany have traced the steps that the motor protein kinesin takes in more detail than ever before by getting it to carry a germanium nanosphere.
Kinesins play a vital part in many living cells, including in humans’, dragging cargos like neurotransmitter-filled vesicles along microtubule rails. Kinesins pull with piconewton forces, which Erik Schäffer’s from the University of Tübingen likens to a millionth of a millionth of the gravitational force acting on a chocolate bar.
Scientists first focussed optical tweezers on silica beads attached to kinesin proteins in 1993 to measure the forces they experienced. Breaking down one molecule of the biological fuel adenosine triphosphate (ATP) powers the protein to take an 8nm step forward. Later, researchers claimed to see 4nm substeps, but others disagreed. ‘Our aim was to ask, can we push the limits to actually properly resolve these substeps?’ Schäffer recalls.