Victoria Richards investigates the world of artificial molecular machines – where have they come from and where are they headed?
What if we lived in a world run by molecular machines, too small to see but impacting all aspects of our everyday lives? In one sense, we already do: biology uses them for absolutely everything – from harvesting energy from the sun to the way that we see, with proteins being the most complicated of the lot. Scientists have taken to calling them machines because, just like those designed by humans, they produce mechanical motion in response to an input, allowing them to perform a task. Whereas biology has perfected its machines over billions of years of evolution, chemists keen to imitate these structures are just getting started.
In the late 1980s, researchers started building assemblies of molecules that contain mechanically interlocked components, where two or more parts can’t be separated without breaking a covalent bond. The most popular of these came to be the rotaxane – a dumbbell-shaped molecule with a ring wrapped around the centre, free to slide along the axle but too small to come off at either end. ‘The credit for making molecular machines attractive to chemists goes to Fraser Stoddart,’ recalls David Leigh of the University of Manchester, UK. ‘He had the vision to realise that these architectures gave you the possibility of large amplitude-controlled motions, and that that could be the basis of molecular machines.’
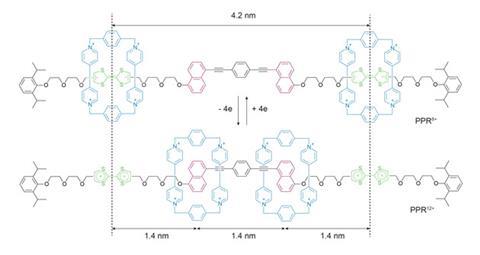
Just over a decade ago a team in the US led by Fraser Stoddart, now at Northwestern University in Illinois, showed that they could use rotaxanes to bend gold.1 They used redox chemistry to control the position of a macrocycle that shuttles back and forth along a thread between two stations: in the neutral state the macrocycle has a high affinity for a tetrathiafulvalene unit, but on oxidation it is repelled to sit much more comfortably on a naphthalene station. When two of these rotaxanes are coupled together, the rings find themselves either 4.2 or 1.4 nm apart depending on the redox state. By anchoring the macrocycles to a gold surface, the group were able to mimic the kind of contractions and extensions that you get in muscles and use these switches to bend micron-sized gold beams.
Reinventing the wheel
Stoddart’s study showed that molecular machines could influence objects many magnitudes larger than themselves, but looking beyond switches, scientists have since moved on to designing more complex motor-based systems.
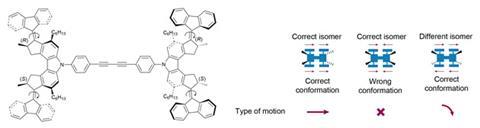
The nanocar – a four-wheeled molecule that drives across a copper surface – made the news in 2011.2Ben Feringa and co-workers at the University of Groningen in the Netherlands designed a motor that rotates in a single direction thanks to a series of conformational changes. Electronically exciting their molecule causes a photochemical isomerisation around a carbon–carbon double bond, and a helix inversion quickly follows. Two cycles of these geometric changes give a 360° rotation of the motor in a paddle-wheel type movement. Substitute four wheels for motors and when all of them are aligned to spin in the same direction, the molecule propels itself forward in a straight line.
‘The entire regime of motion in the molecular world is completely different to in the macroscopic world, and so what people call nanocars have nothing at all to do with the physics of a car,’ comments physicist Dean Astumian at the University of Maine, US. ‘It’s like when you look up in the night sky and see a constellation that looks like a bear: we wouldn’t think that the biology of a bear is useful for understanding how the stars in that constellation move relative to one another,’ he adds. Regardless of the questionable comparison, there’s no doubt that achieving this type of molecular directionality is no mean feat, especially given that molecules usually move around constantly and at random.
It’s also well worth considering that it’s this random motion that allows molecular machines to function at all. ‘We all know that Brownian motion cannot serve as a source of energy, so thermal noise cannot provide energy to drive directed motion,’ explains Astumian. ‘But you can have motors that work using noise, which even if you provided energy, without noise would not work at all’. Indeed, without thermal noise the macrocycle in Stoddart’s switch would stay put regardless of redox state, and the whole system would become useless. Taking advantage of Brownian motion and harvesting it to achieve a desired movement is therefore key when it comes to designing molecular machines.
Strolling down a molecular track
‘Rather than trying to downsize engineering concepts from the big world to the small world, to me it seems better to try and learn how biology copes with engineering machines at that length scale and apply those concepts to making our own molecular machines,’ Leigh explains. Flicking through biology’s catalogue of machinery for inspiration, Leigh’s team designed a two-legged linear motor and got it to walk along a track,3 mimicking the way that linear motor proteins move down intracellular tracks to perform tasks.
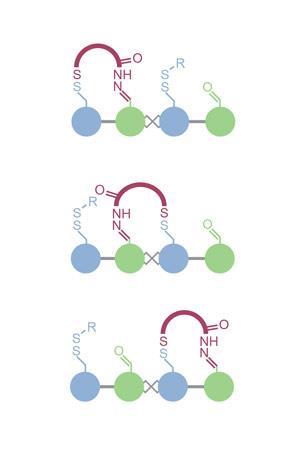
The walker uses a combination of acid–base chemistry and photochemical isomerisation to get from A to B. As gravity is negligible in the molecular world, one foot must be locked in place so that the molecule doesn’t fly off the track. When one foot is pinned down, the other foot has to be able to step forward, and then the chemistry must change to lock the second foot in place to take the next step. The team use disulfide bonding on one foot and hydrazone bonding on the other, so that one is kinetically locked in acid while the other is labile, and vice versa in base; oscillating the pH in the system then allows the molecule to walk up and down the track. Getting the walker to go in any particular direction is a little trickier, and that’s where photochemistry comes in – isomerising a built-in stilbene unit on the track induces ring strain in the motor and entices it to move in one direction over the other.
‘We’d like to be able to carry molecules along tracks to particular parts; for example, picking up reactive building blocks and taking them along a track to an assembly site and constructing a molecule,’ says Leigh. ‘Imagine you’ve got branched tracks so you can chose where the molecule goes – left track to pick up one amino acid, right track to pick up another. Introducing dynamics and the possibility of spatially addressing different things are going to be very useful at the molecular level, and something that we can’t do any other way.’
His team very recently took steps to achieving just that, building a robotic arm that can pick up a small molecule, swing around on its axis, and release it 2nm away from where it started.4 They use the same dynamic chemistry as for their walker, with a disulfide bond gripping or releasing the cargo, and a hydrazone linkage securing it to the platform. Manipulating molecules using nanorobotics could spark the beginning of synthetic factory-style assembly lines, passing molecules down a chain from one arm to another to build larger structures, similar to how enzymes construct fatty acids.
Pump it up
Stoddart is also a fan of nature’s nanomachines, and he took inspiration from carrier proteins for some recent work. Take bacteriorhodopsin, for example. It pumps protons across a cell membrane against a concentration gradient, and once inside the cell the protons are converted into chemical energy by transforming adenosine diphosphate into adenosine triphosphate. ‘These motors work away from equilibrium and that’s where there’s an enormous challenge to the chemical community,’ says Stoddart. ‘We have been almost born and brought up on equilibrium dynamics – that everything will reach equilibrium and that’s the end of it – but effectively the whole basis for life is working away from equilibrium, pushing systems energetically uphill.’
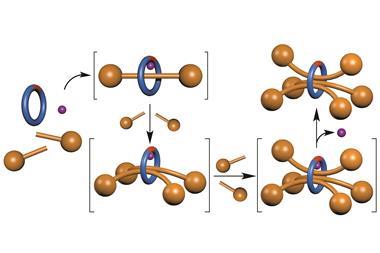
His team designed a rotaxane-based motor that can pump macrocyclic rings onto a thread against a concentration gradient,5 building up potential energy in the system, which could in principle be converted into useful work on the surrounding environment. As energy consumed at the molecular level is typically dissipated through thermal relaxation, examples of artificial machines that exist in such high energy states are extremely rare.
The pump uses redox chemistry as the stimulus and operates using a ratchet mechanism similar to that of Leigh’s walker, except that this time electrostatic interactions are key to forcing the macrocycles to one side. The thread can be divided into two main parts – the viologen unit, with which the macrocycle forms a stable radical complex when the system is reduced, and the collecting chain, on which the macrocycles accumulate when the system is oxidised and the tetracationic ring is repelled away from the viologen pump. The combined effects of the positively charged viologen unit and an adjacent pyridinium group prevent the macrocycle from dethreading when the system is first oxidised, and a gate stops any rings already on the collecting chain from coming back off when the cycle is reinitiated.
‘We’re aiming towards being able to put a multitude of rings onto polymer chains, leading to films that are scratch resistant,’ says Stoddart. ‘If you made a scratch on a surface made of that kind of material, quite quickly the polymer would come and fill up the cracks, presumably because the rings are sliding around on that polymer chain. We’re hoping to take polymer chemistry into a new realm of potential application.’
Getting wound up
Stoddart isn’t the only one with that goal in mind. Nicolas Giuseppone at the University of Strasbourg in France and his colleagues have also been taking advantage of polymers to make high energy materials. The team incorporated rotary motors into an entangled polymer network, and their activation caused mechanical winding of the polymer chains.6 ‘These motors were developed by Feringa, but our approach was to use this basis to produce useful work at the macroscopic scale,’ explains Giuseppone. Coiling of the polymer at each motor causes local contractions, and these are amplified throughout the material causing an overall shrinkage of the gel. For the first time, collective nanometre-sized movements of molecular machines were able to produce mechanical work at the centimetre scale. What’s more, the light energy consumed is stored as elastic energy inside the material, and the team are working on ways to unwind the coils to make use of this energy.
We’re on a very early part of a very steep learning curve
‘How we get from the molecular world, through the nanoscopic and the mesoscopic, to the macroscopic is certainly a major area for development,’ comments Stoddart. ‘The first thing to do is to put molecular machines into an ordered environment, and then there’s the whole process of working them into polymers so that the machine is repeated n times over.’ While Giuseppone’s motors certainly repeat within a polymer, they are not ordered in two or three dimensions, a requirement if molecular switches or motors are ever to break into the world of computing.
To that aim, a team led by Stephen Loeb at the University of Windsor in Canada built a metal–organic framework that incorporates a rotaxane within its linker, making the shuttle perfectly repeated inside a three-dimensional lattice.7 ‘We have demonstrated the basic translational motion; the next step is to be able to control that motion with some kind of stimulus, the same way people have done in solution,’ says Loeb. ‘I think we’re fairly close to that – the hard part is learning to organise these things so they can at least move in the solid state – the roadmap to controlling motion is already there from the solution chemistry.’
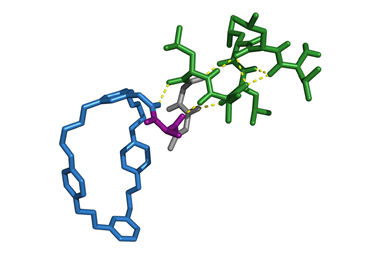
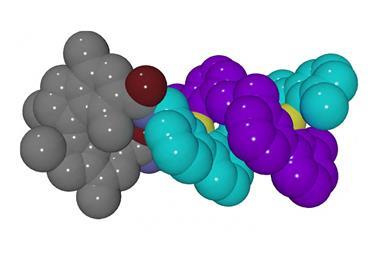
While scientists like Stoddart and Giuseppone are convinced that artificial machines will find their calling in macroscopic work, others are less so. ‘My perspective has always been that it’s much more interesting to challenge molecular machines with molecular tasks,’ says Astumian. Sharing that sentiment, Leigh and co-workers built a ribosome mimic that can make a peptide with a pre-determined sequence, and it’s arguably the most sophisticated molecular machine around.8 They designed a rotaxane covered in the different parts that need to work together to get the job done. Their thread hosts a number of amino acids in a desired order, and their macrocycle, carrying a catalytic arm, slides along the axle until its path is blocked by an amino acid. A thiolate group on the arm then picks up the amino acid and hands it over to an elongation site further up the limb. The macrocycle then goes along its way until it reaches the next amino acid, where the same thing happens again, until none are left and the ring falls off the other end. Put a trillion of these machines to work at once and they churn out tens of milligrams of peptide, made up of only a single sequence.
‘It’s still a really simple thing. To compare it to the ribosome which is infinitely better than our molecule is a bit of a joke – all we can do is put together a few peptide bonds, whereas the ribosome does fifteen a second,’ says Leigh. ‘Still, the ribosome has about a million atoms in it and ours has got a few hundred. So we can mimic something that’s absolutely enormous with a very stripped-down synthetic system, and we’re very excited about that conceptually.’
While molecular machines have come on leaps and bounds over the last decade, it’s still not clear what they might actually end up being useful for. Stoddart isn’t too worried about what the future holds: ‘We’re on a very early part of a very steep learning curve. Chemistry is a fundamental science and it needs some space in which to develop the fundamentals. It’s going to be a slow process and it may take decades to develop the field to a stage where it’s applied to whatever the technology of the day is, but then suddenly it will take off, and people will see what all that fundamental development can lead to.’
Leigh’s convinced that what the field needs is a killer application. ‘As soon as you find one thing that molecular machines can do that can’t be done any other way, then they undeniably become a useful technology. Biology has found through evolution that molecular machines are the best way to get things done. I’m sure that’s going to be true for mankind as well.’
Victoria Richards is a science writer based in Cambridge, UK
References
1 Y Liu et al, J. Am. Chem. Soc., 2005, 127, 9745 (DOI: 10.1021/ja051088p)
2 T Kudernac et al, Nature, 2011, 479, 208 (DOI: 10.1038/nature10587)
3 M J Barrell et al, Angew. Chem., Int. Ed., 2011, 50, 285 (DOI: 10.1002/anie.201004779)
4 S Kassem et al, Nat. Chem., 2015, DOI: 10.1038/nchem.2410
5 C Cheng et al, Nat. Nanotechnol., 2015, 10, 547 (DOI: 10.1038/nnano.2015.96)
6 Q Li et al, Nat. Nanotechnol., 2015, 10, 161 (DOI: 10.1038/nnano.2014.315)
7 K Zhu et al, Nat. Chem., 2015, 7, 514 (DOI: 10.1038/nchem.2258)
8 B Lewandowski et al, Science, 2013, 339, 189 (DOI: 10.1126/science.1229753)







No comments yet