No longer valued simply for its glamour and durability, diamond is turning its hand to applications in solar power, laser design and bionic eyes. James Mitchell Crow reports
Humans have long been drawn to diamonds. And not just for their sparkle. Since its earliest discovery, people seem to have appreciated diamond’s material properties just as much as its aesthetic charms.

But recent work by physicist Peter Lu from Harvard University in Boston, US, has pushed back the likely date of diamond’s first use to at least 4500 years ago - and again, it wasn’t for their glitter that the diamonds were being used.
Lu and his colleagues were studying a series of highly polished, almost lustrous ceremonial axes that had been found in the tombs of wealthy individuals from the Neolithic period in China. Using x-ray diffraction and scanning electron microscopy, the team showed that the main component of the axes was corundum, a crystalline form of aluminium oxide that is the second hardest material found in nature. The most likely material for polishing such a material to a mirror-like sheen is something even harder, the team concluded - which can only mean diamond.
The ancient Chinese appear to have been tapping what is now one of diamond’s best known properties. The hardness of diamond comes from the particular arrangement of its component carbon atoms. Unlike the planar carbon sheets that make up graphite, in diamond the atoms form a cubic lattice structure in which each is covalently bonded to four others in a tetrahedral arrangement.
Not just a pretty face
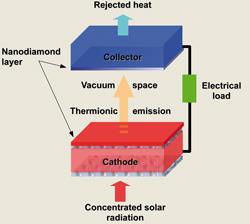
We now know that diamond’s exceptional properties extend far further than hardness. Its network of strong covalent bonds also makes it the most thermally conductive material of any solid known. It also happens to be an excellent low temperature thermionic emitter - when heated, electrons boil off from its surface.
A team at the University of Bristol, UK, is looking to capture those ejected electrons and put them to good use by developing a diamond-based solar panel. The researchers won a €1 million (£870,000) grant from energy company E.ON to build a working device. The three year project ends this October, and all the components have now been developed, says Neil Fox, who is leading the project. ’This August we’ll be putting it all together.’
The device will look something like a satellite dish. A parabolic reflector will focus solar energy onto a thin layer of diamond particles, which will eject electrons across a narrow vacuum to a collector electrode, from which they will make their way around an electrical circuit. A big part of the project has been to drive down the temperature at which thermionic emission starts, from around 400°C to 350°C or below, to make solar energy harvesting as efficient as possible. Fox’s trick is to form a lithium monolayer on the diamond’s surface, creating a dipole that helps to kick the negatively charged electrons off the surface. ’We’re hoping that we can achieve amps of current [from our prototype],’ says Fox. ’We’ve already had glimpses.’
Made to measure

As well as heat, diamond also has some interesting tricks when it comes to interacting with light. A team at Macquarie University in Sydney, Australia, is starting to develop the first diamond-based Raman lasers. ’On paper, diamond looked like it had some really good properties,’ says Rich Mildren, who leads the work. ’But we didn’t know how it would actually perform.’
Raman lasers work by stimulated light scattering, an effect that is particularly strong in diamond, thanks in part to its strongly bonded and highly symmetric structure. As for many applications of the material, what is finally allowing such properties to be tapped is the increasingly sophisticated ways in which high quality diamond can be grown. Whereas every diamond dug out of the ground is unique, with its own flaws and impurities, growing diamond allows consistency. Traditional high pressure, high temperature approaches are beginning to be replaced by chemical vapour deposition (CVD), in which carbon-based gases such as methane are converted to diamond on a surface. CVD gives much greater control over how the diamond grows.
’It’s in the last 10 or 15 years that we’ve been able to nanostructure diamond to meet particular requirements,’ says Steven Prawer, who heads the Melbourne Materials Institute at the University of Melbourne, Australia. ’Our ability to grow it in a particular shape and with particular properties is what has opened up diamond as an engineering material.’
Lighting the way
The progress in growing high purity, low defect diamond is certainly helping Mildren. ’We’ve been quite lucky so far, diamond does look to be a very good laser material,’ he says. Their first success has been in developing what promises to be the first practical and cost effective laser to emit yellow light. It’s a wavelength that has important potential medical applications, from treating certain skin conditions to biomedical imaging.
’We’re excited about pushing the research further to try to demonstrate diamond lasers that can perform much better than other materials,’ Mildern adds. The possibilities include very high power Raman lasers - diamond’s strength and thermal conductivity means that it can handle high power without melting or fracturing - and lasers that emit light at wavelengths that other materials can’t, such as into the far infrared.
Prawer is also looking into diamond’s interactions with light, this time at the level of single photons. Single photon emitters are of great interest for secure communication technologies on which it is impossible to eavesdrop, says Prawer. ’How do you do that? You rely on the laws of quantum mechanics.’
’If we send messages just one photon at a time, then that photon carries with it quantum information,’ he explains. ’If someone tried to eavesdrop, the state of the photon is changed, and you instantly know that someone is listening in.’
The difficulty is in reliably generating single photon pulses, which is where diamond comes in. ’Diamond has a unique property: if you introduce a single nitrogen atom impurity, you create a defect that shines extremely brightly - but because it’s just one atom, every time it emits light it is just one photon.’ Prawer and his colleagues have also created the necessary optics and electronics around the diamond crystal to turn it into the first commercial single photon source system, which is now being investigated for secure communication applications.
Extreme environments
To a chemist, a material that is highly stable might not sound that appealing. But it is one of the key properties in many of diamond’s applications, including being sent to Venus as part of Nasa’s Pioneer Venus mission. Venus has a highly corrosive atmosphere, with swirling clouds of sulfuric acid. Surface temperatures and pressures on the planet can reach over 450°C and 100atm.
The scientific instruments on board the Pioneer Venus Orbiter, which circled the planet from July 1980 until May 1992, when it was sent into the Venusian atmosphere, were protected within titanium spheres. But instruments such as the infrared radiometer, which measured IR emissions, needed windows through which to sample their surroundings. No glass or plastic could survive the corrosive, high temperature environment, so diamond was selected, which is infrared transparent. Two tiny windows were fashioned from specially selected natural diamond. Today, such windows can be synthesised routinely using CVD.
Closely related to diamond’s chemical inertness is its biocompatibility. This property has been shown to be retained right down to the nanoscale - unlike other nanoforms of carbon such as carbon nanotubes, whose biocompatibility remains a subject of debate. It’s a property that is starting to see diamond being used in the body, from drug delivery systems to medical diagnostics to bionic implants.

This year, a team in the US showed how nanodiamond-based drug delivery has the potential to overcome drug resistant cancers, including breast and liver cancer. Many tumours resist treatment not because anticancer drugs aren’t active, but because cancerous cells actively pump the drug out of the cell before it can take effect.
To get around this problem, Dean Ho from Northwestern University in Evanston, US, and his colleagues turned to nanodiamond as a drug delivery vehicle. Nanodiamonds are typically made by detonation. Exploding a mixture of trinitrotoluene (TNT) and cyclotrimethylenetrinitramine (more commonly known as RDX) produces a soot riddled with 2-8nm diamond particles. The surface of diamonds produced in this way tends to incorporate a range of functional groups - carboxylic acids, for example - that can be used as a handle to attach molecules of interest, such as doxorubicin.
Ho decorated his diamond nanoparticles with the anticancer drug doxorubicin, essentially turning them into a slow release drug delivery system. The cells were unable to eject the nanodiamond, resulting in prolonged activity of the drug and a higher rate of cancer cell death. ’Our results show nanodiamond’s enormous potential towards significantly improving the efficacy of drug resistant cancer treatment and simultaneously improving safety,’ says Ho.
The work follows closely on the heels of a study by Ho’s colleagues at Northwestern that demonstrates nanodiamond’s potential in bioimaging applications. Thomas Meade and his team used diamond as a carrier for a gadolinium contrast agent in magnetic resonance imaging (MRI) studies. MRI works by detecting the proton NMR signal of water molecules in different tissues of the body, an effect enhanced by gadolinium.
By attaching the gadolinium to diamond, the team boosted the signal enhancement per gadolinium atom - a factor the team suspect is down to the nanodiamond itself, as it is known to attract water molecules onto its surface.
A real eye-opener
For other applications, diamond comes to the fore not because it excels in a single area, but because the combination of properties it possesses is unique. ’It’s when you need something that is very hard and very biocompatible, very stable and has tuneable electronic properties that diamond fits the bill,’ says Prawer.
This is the combination of properties crucial to the latest project that Prawer is working on. He and his colleagues are developing implantable diamond electrodes that could soon be allowing the blind to see again - at least to some degree.
Prawer is part of the project called Bionic Vision Australia, which is developing a bionic eye. At the heart of the device is a chip that will be implanted on the retina. Images captured by a camera attached to a user’s glasses will be transmitted to the chip, which will convert these data into electrical impulses to stimulate the optic nerve.
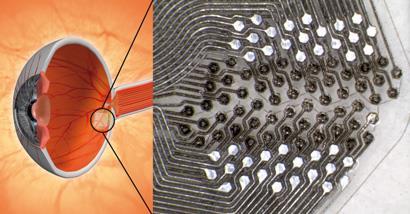
The device will work for people whose blindness is caused by lost photoreceptors, but whose neurons within the eye are still intact. Diseases that fall into this category include age-related macular degeneration (AMD), which is the leading cause of vision impairment among older people, and retinitis pigmentosa, which affects one in 3000 live births worldwide.
Diamond is being used for several essential components of the implanted chip, which is the part of the project that Prawer is leading. His team is exploiting the fact that by doping diamond as it is grown, it can be turned from an electrical insulator into a conductor. They are growing diamond into high density electrode arrays that will penetrate the retina and stimulate the neurons located there.
While the data being fed to the brain by the system will be a fraction of that captured by a healthy eye, earlier work with cochlear implants suggests that the brain will be able to put together a surprisingly good image. ’The brain is extremely plastic,’ says Prawer. ’It can put limited information to good use.’
Tests suggest that diamond is particularly well suited to life in the eye - so the team are now also developing a diamond casing, 50um thick, to protect the electronic components of the implanted chip. ’By the end of 2013, we’ll be ready to implant the array into animal models,’ says Prawer. ’If the implants are as successful as the cochlear implant has been, it won’t be long before we can have people reading using these implants.’
James Mitchell Crow is a freelance science writer based in Melbourne, Australia












No comments yet