Total synthesis – Page 5
-
-
-
 Opinion
OpinionAn 'Aye' for details
Small steps by synthetic chemists could mean giant leaps for those who follow, says Karl Collins
-
Opinion
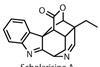
Pactamycin
Labelling a molecule 'inaccessible to synthesis' is a red rag to a bull, says Paul Docherty
-
Opinion
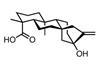
Pentalenolactone A methyl ester
Paul Docherty revises a reaction he never could quite remember
-
 Feature
FeatureStepping toward ideality
James Mitchell Crow wonders what would make the perfect organic synthesis
-
Opinion
atrop-Abyssomicin C
Natural products frequently have hard to pronounce and even harder to spell names, often of little meaning.