Evidence mounts that water has two liquid forms
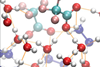
Supercooled solution reveals phase transition at -80°C
Are there two forms of liquid water? A debate has raged ever since that idea was first proposed over 25 years ago. A new study now adds to the evidence that there may indeed be two. It reports direct experimental evidence for a phase transition between two liquid states – but in an ionic solution, not in pure water.1 The researchers say, however, that the state of the water in the mixture is much like that in the pure bulk liquid, strongly suggesting that water itself might show similar behaviour.
