The potential presence of phosphine on Venus is only the latest twist in the strange chemistry of our planetary neighbour, finds Clare Sansom
About 4.5 billion years ago three similar smallish rocky planets formed around our Sun. Had any interstellar visitors been watching the solar system at that time, however, they could not have guessed at their radically different fates. While our planet developed ideal conditions for complex biology to evolve, neither Mars nor Venus was able to hold on to either of the two most important pre-requisites for life: atmospheric oxygen or liquid water. In fact, scientists are split over Venus’ past; some believe that it might once have had oceans and, perhaps, been habitable, while others think its water could never have condensed.
Both our planetary neighbours have fascinated stargazers for millennia. Attention has often focused on whether life might exist on either, whether it might have existed there in the distant past and whether it might evolve in the far future. What we know of the planets’ atmospheres essentially rules out anything resembling the Martians or Venusians of HG Wells, or indeed any other science-fiction writer, but bacteria, which exist almost everywhere on Earth, might perhaps be found even there.
Contemplating Venus’ cool light and the imagery of the ‘planet of Love’ that has echoed through the centuries, poets would never imagine the extraordinary hostility of the environment at its surface. These apocalyptic conditions, with temperatures of over 450°C, an atmospheric pressure over 90 times that on Earth, exceptional acidity and no liquid water, are many times harsher than those needed to immediately fry even the hardiest of the Earth’s extremophile bacteria.
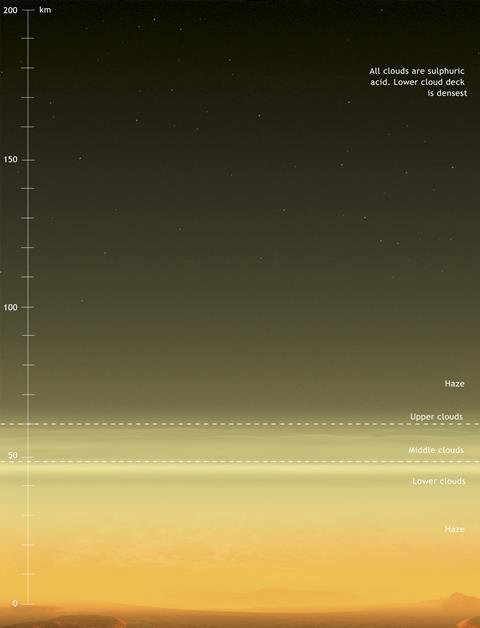
Many miles above its surface, however, we meet conditions that are among the most benign of those found in the extra-terrestrial solar system. Venus’ lower atmosphere is thick, hot, and dominated by thermochemistry; its upper atmosphere is cold and dominated by photochemistry, rather like the Earth’s upper atmosphere; but the cloudy layer between the two at heights of about 50km above the surface is more interesting. It is temperate (~20°C) and acidic, at close to our atmospheric pressure. At this altitude, a human astronaut would need neither a pressure-controlled nor a thermally controlled space suit: brief excursions outside a balloon-borne craft could be carried out with only an oxygen mask and perhaps a thin layer to avoid sulfuric acid deposition. Therefore, when scientists (rather than science-fiction writers) talk about ‘life on Venus’, they really mean ‘life around Venus’: the serious possibility that forms of bacteria with metabolic pathways that can take advantage of anoxic, hyper-acidic conditions might have evolved to float in that atmosphere.
Enter phosphine
Detecting microscopic life around another planet seems like an almost impossible task. However, planetary atmospheres can be readily analysed from Earth using spectroscopy, and any gas that is observed in the atmosphere of a planet but has no clear chemical, abiotic pathway to its synthesis in the conditions found there can be considered a ‘biomarker’ of the possible presence of living organisms. But not every potential biomarker gas is observable, as Martin McCoustra of Heriot-Watt University in Edinburgh, Scotland, explains. ‘The oxygen in the Earth’s atmosphere has a biological origin, so we might think that observing it in another planet’s atmosphere would be a sign of life. It is, however, difficult to observe spectroscopically; ozone (O3) is easier, but abiotic mechanisms for producing molecular ozone exist so it is not ideal as a biomarker.’ And neither oxygen nor ozone has, in fact, been observed in Venus’ atmosphere in any significant quantities. But there are other gases that fit the biomarker specification much better, including phosphine (PH3). A Nature Astronomy paper in September 2020 suggesting that this gas had been found in Venus’ cloud decks caused excitement and controversy far outside the astrochemistry and astrobiology communities.
There are no known abiotic paths to phosphine synthesis in Venus’ oxidising atmosphere
Phosphine is an unpleasant gas that no one would automatically associate with life. It is toxic, flammable and explosive, with an odour that is reminiscent of decaying fish. It can be synthesised spontaneously only in reducing atmospheres such as those around the gas giants Jupiter and Saturn; wherever it is found on Earth, it is associated with human industrial processes or with bacteria. ‘There are no known abiotic paths to phosphine synthesis in Venus’ oxidising atmosphere,’ explains Jane Greaves from the University of Cardiff in Wales, first author on the paper. ‘Furthermore, we have exact knowledge of one phosphine absorption wavelength to less than one part in 107, so we can readily distinguish between phosphine and anything else; these two features combine to make the gas an ideal biomarker to choose for investigating Venus.’
The experiments that led to the controversial Nature Astronomy paper began in 2017, when Greaves and her team were awarded time on the James Clerk Maxwell radio telescope in Hawaii, US. This is the largest single-dish telescope in the world that is designed to observe the millimetre and submillimetre part of the electromagnetic spectrum, where phosphine’s absorbance line at 1.123mm is located. Much of the group’s time since then has been spent in complex data analysis. ‘Venus emits a lot of radio signal that will bounce off the floor or the dome of a telescope, so you end up observing the same signal at a different time, and this can be interpreted as a different signal,’ explains Greaves. ‘We were of course delighted when a statistically significant signal at that wavelength finally emerged from the data.’ A similar experiment using the Atacama large millimetre/submillimetre array (Alma) in Chile also suggested the presence of a line at the same precise wavelength.
This extraordinary claim required extraordinary evidence
Greaves’ analysis remains controversial, however. Her decision to release all the data into the public domain for further analysis has already generated papers on both sides of the debate. Mark Thompson, a molecular spectroscopist at the University of Hertfordshire in the UK was one of the first to publicly question the phosphine result. ‘The discovery of such a reduced molecule as phosphine in the oxidising atmosphere of Venus would be, at least, unusual … this extraordinary claim required extraordinary evidence,’ he says, pointing out that the data Greaves released did not, in his view, meet these ‘extraordinary’ criteria. He quotes the great mathematician John von Neumann to illustrate the pitfalls of data interpretation through complex curve fitting: ‘With four parameters, I can fit an elephant and with five I can make him wiggle his trunk’.
One of the many reasons why this data is proving so difficult to analyse is the low signal to noise ratio; Venus’ background radiation is so bright that individual spectral lines are swamped, as they would if you were to take a very sensitive spectrum of a searchlight. ‘We would need some of the best weather ever recorded at the Alma telescope to record any more phosphine lines,’ adds Greaves. The dry, clear micro-climate at Alma on the high-altitude Atacama Desert plateau is already more suitable for radio astronomy than that of almost any other location on earth.
In the flurry of papers, many still preprints, looking at the phosphine data, some have been unable to see the spectral lines using different analysis, while others attribute it to sulfur dioxide. It’s clear that more – and better – data is needed. But where from?
Sending spacecraft
Radio spectroscopy is not the only technique available for probing the chemistry of Venus’ atmosphere. Much can be learned from spacecraft orbiting the planet, and it is possible to obtain measurements both from its extremely hot surface and from probes descending through the atmosphere. The Soviet Union’s Venera missions to Venus in the 1960s and 1970s were the first to orbit, and then to touch down on, any other planet and to return photographs and data. Nasa’s Pioneer missions followed soon afterwards, but it was widely recognised that the Soviets had won the Cold War race to Venus, just as the US, with its Mariner missions, had won the race to Mars (and to the Moon).
Many exoplanets will likely turn out to be more Venus-like than Earth-like
Some Venusian missions continued into the 1980s, but the end of the Cold War coincided with the beginning of a long gap: the only spacecraft sent to Venus between 1990 and 2004 were flypasts, such as the US Cassini probe on its way to Saturn in 1998 and 1999. ‘During the 1990s and early 2000s the community focused on Mars as the more “obvious” target for possible human exploration,’ says Colin Wilson of the University of Oxford, UK, who is working to develop the European Space Agency’s proposed EnVision mission to Venus. ‘Our interest in Venus grew again due to our rising awareness of the greenhouse effect as a driver of climate change, and also to a growing interest in exoplanets, many of which will likely turn out to be more “Venus-like” than “Earth-like”.’
The hostile conditions on Venus’ surface provide unique challenges to the designers of scientific instruments. At the temperatures found there, solder will melt, and all conventional electronics fail. ‘Short-duration landers such as the Venera 14 mission in 1982 are passively cooled and have about an hour on Venus’ surface to collect data before their equipment fails,’ says Wilson. ‘There are essentially two alternatives for longer-duration landers. Heat pumps can be used to cool the electronics, but this is complex and energy intensive. Alternatively, silicon carbide electronics can operate at the Venusian surface temperature, but this is still fairly primitive.’ Power sources are also problematical: the dense clouds and high temperatures make solar cells unviable, and the batteries that can be used have a limited capacity. However, not all experiments require landers; others are carried by balloons released from orbiters into the cloud layer. These can ride Venus’ high winds to circumnavigate the planet in as little as five days, and the equipment they carry needs to endure only the benign, more Earth-like conditions found at that altitude.
The data sent back to Earth by these landing crafts has already provided some important insights into the chemistry at Venus’ surface. It is an active planet, with volcanic structures like those on Earth but bigger and hints of current volcanic activity. Measurements of the elemental composition of rocks from these volcanoes have shown that they are similar to common terrestrial basalts.
There is only one spacecraft operating at Venus at present – the Japanese Akatsuki orbiter, whose meteorology-oriented instruments have little capability to detect trace gases – and no further spacecraft have been selected by space agencies to go to Venus after Akatsuki. Many proposed missions are vying for funding from these agencies, however. Some would descend slowly through the Venusian atmosphere, making sensitive measurements of its composition as they descend. These could study a huge range of chemical processes from sulfur chemistry to halogen chemistry, as well as searching for phosphine. They could also measure the abundance and isotopic ratios of the noble gases. These are so inert that their abundances are unchanged from what they were when the planet was formed, or since they were outgassed from the mantle, so measuring them could tell us more about Venus’ early evolution.
Other missions in development would seek to unravel Venus’ history by studying its geological record, using radar to map the surface of the planet in detail. The EnVision orbiter, which has been proposed to ESA for launch in 2032, would also look for traces of active chemical species, including halogens, water vapour and heavy water as signs of volcanic activity. All these measurements are more likely to suggest whether some forms of life might have existed, or even flourished, on Venus billions of years ago than they are to shed further light on the hypothesis that bacteria now exist in the clouds.
What about us?
How might the phosphine controversy be solved, one way or the other, and preferably within the next decade? If one thing is certain, it is that we need more data. Earth-bound radio astronomy has not yet reached its limits, and it will certainly be possible to search for further phosphine absorbances – although many of these are below 1mm, in parts of the radio spectrum where the Earth’s atmosphere is opaque. ‘You can avoid the opaque atmosphere by launching a telescope into space, but this is technically challenging and incredibly expensive,’ explains Greaves. ‘Phosphine has an absorption spectrum in the infra-red, which might be accessible,’ says Thompson. ‘But so far, at least, none of these lines have been detected.’
Venus demonstrates a greenhouse effect taken to extremes
If we do find evidence of life around Venus or elsewhere in the Solar System, it may help us understand its origins on our own planet. It is possible that the early Mars or Venus might have developed in such a way as to allow complex life to evolve, but instead one lost its atmosphere completely and the other developed a thick, toxic one. Liquid water can exist on neither of our neighbouring planets in sufficient quantities for biology as we know it to exist. ‘The Earth is a special case, a “Goldilocks planet” with conditions exactly suited to support life, but we ourselves are not special: we are just lucky,’ says McCoustra. ‘We can’t go back and watch evolution from the beginning, and the fossil record only begins after unicellular life was established, about 3.5 billion years ago. However, what we are learning about the chemistry of the complex, pre-biotic organic molecules that form outside the Earth suggests that it is possible – although not inevitable – that evolution might be able to repeat itself.’
And studies of Venus, and the simple life forms that might possibly exist there, have implications for our ‘lucky’ planet’s future as well as its past. ‘The surface of Venus is the hottest place in our Solar System, despite the fact that it is further from the Sun than Mercury,’ explains Anthony Meijer from the University of Sheffield, UK. ‘This demonstrates a greenhouse effect taken to extremes, with heat trapped by the very dense atmosphere.’ Most astrophysicists who believe that our own planet will end like this date it to perhaps billions of years in the future, but there are faint echoes here of our own possible futures. Those portrayed in the emerging genre known as climate fiction or ‘cli-fi’, and in authoritative non-fiction such as Storms of my Grandchildren by the climatologist James Hansen, depict a planet Earth rapidly losing its Goldilocks qualities and, perhaps, suggesting the path taken by Venus, billions of years ago.
‘The most powerful words in science are not ‘Eureka’ but “That’s funny…”’ suggests Meijer. The jury is still out on Greaves’ ‘funny’ results, which she describes as ‘anomalous and unexplained chemistry’. Whether or not phosphine is present in tiny quantities in Venus’ clouds, there is much still to discover about our nearest neighbour, and its – and our – past and future. This will involve science, including chemistry, that the EnVision mission, if chosen for funding, should be well equipped to carry out.
Clare Sansom is a science writer based in Cambridge, UK











No comments yet