Venoms are a treasure trove of peptides that may provide a bounty of novel painkillers
Snakes, spiders and scorpions are some of the most lethal animals on the planet, producing venoms either as part of a defence mechanism towards predators, or as a means to subdue and kill their prey for food. For millions of years, spider and scorpion venoms have evolved to disable the central nervous system of insects. In contrast, most snake venoms target the circulatory system of vertebrates, resulting in uncontrolled bleeding and organ failure. And yet, ironically, the properties that make venoms deadly are the same properties that can be used for lifesaving medicines or analgesics.
There are currently six US Food and Drug Administration (FDA)-approved drugs derived from animal venom peptides and proteins, with many more in clinical trials or at various stages of preclinical development. Captopril, for example, based on a pit viper toxin, was developed by US drug company Squibb (now Bristol-Myers Squibb). It’s used to treat high blood pressure and other heart conditions. Captopril was approved by the FDA in 1981 and by the European Medicines Agency (EMA) in 1984; since then it has undoubtedly saved many more human lives than the snakes have taken.1

More recently, Jim Olson and his team at the Fred Hutchinson Cancer Research Center in Seattle, US, have begun investigating ‘tumour paint’ in clinical trials. This paint contains chlorotoxin, a 36-amino acid peptide found in deathstalker scorpion venom. The molecule attaches itself to cancerous brain cells in preference to healthy ones, illuminating them through a fluorescent tag attached to the toxin, thus showing surgeons exactly where to cut during surgery. The paint should help surgeons remove brain tumours more precisely, as leaving behind some of the cancerous cells – or taking away too many surrounding healthy cells – can have devastating consequences for patients.
A painful problem
Animal venoms are also showing their potential in pain relief, enabling a reduction in the amount of opioids given to a patient and even allowing different pain pathways in the body to be targeted.
Chronic pain affects nearly 20% of the population, a percentage that more than doubles by the age of 65. In the US, chronic pain was recently estimated to cost around $600 billion (£450 billion) per year, exceeding the combined economic burden of cancer, heart disease and diabetes.2 When it comes to treating pain, opioids are a popular choice. They are regularly used for treating moderate to severe acute pain, due to their rapid onset and high efficacy. Opioids are also often used to manage the severe, chronic and disabling pain from terminal conditions, such as cancer, and degenerative conditions like rheumatoid arthritis.
These drugs bind to opioid receptors, proteins that are widely distributed throughout the body. Receptors responsible for pain modulation are situated in both the central and peripheral nervous systems. These naturally bind to opioid peptides known as endorphins, whose principal function is to inhibit the transmission of pain signals to the brain.3
There is room to improve on opioids, however. ‘Opioids don’t work that well for many pain conditions,’ says Irina Vetter, deputy director and pharmacologist at the Institute of Molecular Bioscience Centre for Pain Research at the University of Queensland, Australia. ‘For example, if you have nerve damage they don’t work very well. You have to take very high doses and then you get side effects like respiratory depression and constipation, plus they also cause tolerance meaning you have to keep increasing the dose.’ Their use can also lead to problems with opioid dependence and addiction, with ever-increasing reports of this drug being abused and fatal overdoses.
Along came a spider
In 2006, a team led by James Cox at the Cambridge Institute for Medical Research, UK, reported landmark discovery: that the gene SCN9A plays a significant role in human pain perception.4 SCN9A is responsible for encoding a voltage-gated sodium channel (Nav1.7) present in pain-sensing neurons (nociceptors). The team was investigating individuals with a congenital inability to feel any pain, finding that this was caused by SCN9A mutations that resulted in a complete loss of function of Nav1.7. The disruption of this single gene rendered these individuals unable to feel any pain.
If you don’t have Nav1.7 you are incapable of experiencing any kind of pain at all. You are otherwise perfectly normal except for a loss in your sense of smell, because the channel is also found in the neurons used for smelling. This is incredible.
Glenn King, University of Queensland, Australia
‘The study really opened up the field,’ explains Glenn King, a biochemist working alongside Vetter at the University of Queensland. ‘If you don’t have Nav1.7 you are incapable of experiencing any kind of pain at all. You are otherwise perfectly normal except for a loss in your sense of smell, because the channel is also found in the neurons used for smelling. This is incredible.’
Developing drugs to block Nav1.7 function could potentially lead towards novel, safer analgesics. ‘It seemed to be an incredibly exciting target for treating people with chronic pain – if you could find a drug that targets this channel then this drug might be useful for all sorts of pain,’ says King. King’s team has been screening venoms against Nav1.7 using high-throughput fluorescent assays. ‘We have over 600 different venoms in the lab,’ he says. Once his team has found a venom with interesting activity, it then isolates its key compounds.
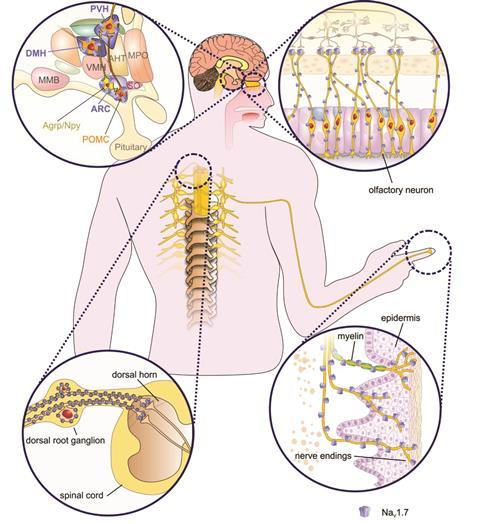
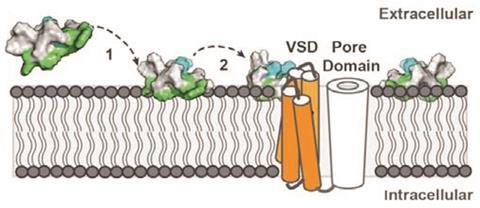
Using this approach, ProTx-II, from the venom of the Peruvian green velvet tarantula, was identified as a selective inhibitor of the Nav1.7. It is one of the most potent Nav1.7 blockers found so far and demonstrates more than 80-fold selectivity over other sodium channel subtypes tested.5 But it isn’t perfect. ‘The problem with ProTX-II is it’s like a piece of grease,’ says King ‘it’s really hydrophobic and sticks to everything and takes a long time to get into the channel and is a slow binder.’
It also isn’t selective enough. We have nine voltage-gated Nav channel sub-types; four are involved in pain signalling. ‘You want to be able to very selectively target that one channel [Nav1.7] because some of the others have very important roles,’ explains King. ‘For example, if Nav1.5, which functions in heart muscle, was blocked the patient would have heart failure, and if Nav1.4, found exclusively in skeletal muscles, was blocked the patient would be paralysed. Even though ProTX-II has a greater than 80-fold selectivity for Nav1.7 it has high potency for those other channels and is therefore lethal in rodents,’ adds King. Sadly, in vivo studies, at lower doses, have also shown it to be ineffective when it comes to treating acute and inflammatory pain in rodent models, via intravenous and intrathecal injections.
when administered in conjunction with sub-therapeutic doses of opioids, Pn3a showed profound levels of analgesia
Vetter, King and collaborators have recently reported a highly selective spider venom peptide, Pn3a, from the venom of a South American giant blue bloom tarantula. Pn3a potently inhibits Nav1.7 with 1000-fold selectivity over all other Nav subtypes.6 ‘This particular peptide is one of the most subtype selective,’ says Vetter, ‘we have dosed animals at quite high doses and have yet to see any side effects at all.’ However, Pn3a also doesn’t show any analgesic effect in some commonly used rodent pain models. But when administered in conjunction with sub-therapeutic doses of opioids, Pn3a showed profound levels of analgesia. An earlier study had also found analgesic synergy between selective Nav1.7 inhibitors and opioids.7
‘To be perfectly honest, we don’t yet understand how that synergy works,’ explains Vetter, ‘we are trying to work it out at the moment.’ But what’s exciting about the work is that ‘it has shown you can drastically decrease the opioid doses you need to get analgesia’, she says. ‘We are doing preclinical toxicity studies and are hoping to be able to take it into human studies eventually.’
Snakes and snails
Researchers are also looking to other channel targets, including acid-sensing ion channels (ASICs), which are expressed throughout the pain pathway, in the central and peripheral nervous system. Eric Lingueglia, a French molecular physiologist from the CNRS Institute of Molecular and Cellular Pharmacology, in Valbonne, and University of Côte d’Azur, has shown that mambalgins – a class of three-finger peptides from the venom of the black mamba snake – can abolish pain through the inhibition of ASICs.8 Results from rodent models showed that mambalgins can be as effective as morphine but with fewer ’side effects. ‘ASICs are important for pain and blocking them can result in analgesic effects,’ says Lingueglia.
‘Based on our earlier research data we thought the mambalgins would need to be taken in conjunction with opioids, as is the case with Pn3a. We were expecting to get strong analgesic effect, which we thought would have been opioid dependent. The surprise in our case was to find an analgesic effect that was opioid independent,’ says Lingueglia. ‘I wouldn’t say that this peptide could lead to the replacement of morphine, just that these are complementary drugs, where in some pain conditions the use of opioids is not so efficient.’

There is already one venom-derived painkiller in use: ziconotide. Originating from the venomous cone snail species Conus magus, ziconotide is used to treat severe chronic pain. Its active ingredient ω-conotoxin first attracted attention during the mid-1970s when Baldomera Olivera, a biologist from the University of Utah, US, wanted to understand the pharmacology behind cone snail stings killing humans. Early studies showed that the Conus venom was a complex mixture of biologically active components, including a large collection of the neuroactive peptides called conotoxins. It wasn’t until decades later that Michael McIntosh, a young research scientist working for Olivera, discovered ω-conotoxin’s medical significance.
ω-Conotoxin potently and selectively blocks N-type voltage-gated calcium channels (Cav2.2) and shows strong analgesic effects in humans when injected directly into the spinal fluid. Although intrathecal administration is far from ideal, it’s the only way for the drug to reach optimal analgesic efficacy while also reducing the potential for serious side-effects, over more traditional routes such as oral or intravenous administration. Ziconotide was approved in the US in 2004 and the EU in 2005, and today is widely regarded as a highly effective analgesic agent for the treatment of severe chronic pain.9
I think we need to become a bit smarter about how we treat pain – it is a little bit naïve of us to think that one drug is going to work for all types of pain
Irina Vetter, University of Queensland, Australia
‘[Ziconotide] is certainly a proof of principle that you can have venom-derived peptide painkillers,’ says Vetter. However, she views intrathecal administration as less than perfect. Her team are seeking to develop drugs that are effective without the need for a spinal injection, or alternatively reduce the number of injections needed to once a week or less.
So, just how close are we to getting to another breakthrough for venom peptides, specifically targeting chronic pain? ‘There are really big differences between different types of pain and what we won’t be able to do is have a magic bullet that will treat everything,’ says Vetter. ‘I think we need to become a bit smarter about how we treat pain – it is a little bit naïve of us to think that one drug is going to work for all types of pain.’ As with any new drug lead it’s about getting enough preclinical data together to prove it’s worth putting the venom peptide through clinical trials. ‘We are convinced our [mambalgin] peptide is a good drug lead,’ says Lingueglia, ‘but there’s a lot of competition for new treatments against pains, or new targets, so you have to show that your leads are interesting and have additional value compared to others.’
Nine venom-derived therapeutic agents are being investigated in clinical trials, against pain and other indications, and many more are in the advanced stages of development. There are also an enormous number of interesting molecules in animal venoms that have yet to be identified and characterised. It’s only a matter of time before another one obtains approval from the likes of the FDA or EMA.
In the meantime, venoms will continue to play an important role in identifying and understanding pain pathways. It’s time we started to see fearsome venomous creatures in a new light – albeit from a safe distance.
References
1. G F King, Venoms to drugs, Royal Society of Chemistry, 2015
2. S Yang et al, Proc. Natl. Acad. Sci. USA, 2013, 110, 17534 (DOI: 10.1073/pnas.1036285110)
3. A Rosenblum et al, Exp. Clin. Psychopharmacol., 2008, 16, 405 (DOI: 10.1037/a0013628)
4. J Cox et al, Nature, 2006, 444, 894 (DOI: 10.1038/nature05413)
5. V Srivastava, Peptide-based drug discovery, Royal Society of Chemistry, 2017
6. J Deuis et al, Sci. Rep., 2017, 7, 40883 (DOI: 10.1038/srep40883)
7. M Minett et al, Nat. Commun., 2015, 6, 8967 (DOI: 10.1038/ncomms9967)
8. S Diochot et al, Nature, 2012, 490, 552 (DOI: 10.1038/nature11494)
9. K Akondi et al, Chem. Rev., 2014, 114, 5815 (DOI: 10.1021/cr400401e)













No comments yet