Neil Withers pays tribute to Harry Kroto, the co-discoverer of buckminsterfullerene, who died recently
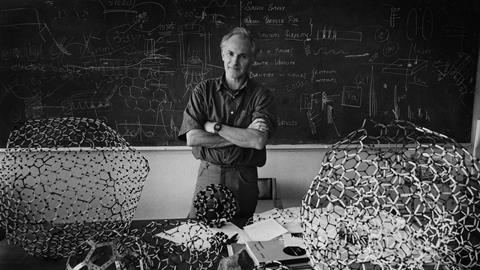
Harry Kroto, who died on Saturday 30 April 2016, was ideally prepared to discover C60, the spherical molecular allotrope of carbon which is the main legacy of his long and successful scientific career. In addition to his scientific interests in spectroscopy, astrochemistry and unusual carbon compounds, he had a keen interest in art and design. He had seen Buckminster Fuller’s geodesic domes at the 1967 Montreal Expo during a postdoctoral fellowship in Canada, and was on the verge of writing to the visionary architect asking for a job before the University of Sussex offered him an academic position.
After winning the Nobel prize and being knighted in 1996, Kroto became the archetypal ‘Nobel prize winner’. He was devoted to inspiring the next generation of scientists, and to preserving curiosity-driven research for the current crop.
These themes – inspiration, curiosity, a wide range of interests and an evangelical zeal for spreading the science bug – summed up Harry Kroto. In 2015, Chemistry World spoke to him at a two-day meeting to mark 30 years of fullerenes.
Berlin to Bolton
With another refugee crisis facing Europe, it is salutary to remember that Kroto’s parents came to the UK as refugees from Nazi Germany. Kroto’s father, then with the surname Krotoschiner, was interned as an enemy alien on the Isle of Man. After the war, he set up a balloon-making and -printing factory, this time in Bolton rather than Berlin. The young Harry spent school holidays in the factory, doing everything from mixing dyes to fixing machinery. Looking back in his Nobel autobiography, he considered this ‘an outstanding training ground for the development of the problem-solving skills needed for a research scientist’.
At school, Kroto wasn’t top of the form. But in the sixth form at Bolton School, he was inspired by his chemistry teacher, Harry Heaney, who would go on to re-enter academia as a professor at Loughborough University. On Heaney’s recommendation, Kroto applied to the University of Sheffield to study for a degree in chemistry.
The work that gives me most intellectual satisfaction is not C60 – although it was a nice experiment
At university, as well as getting first-class degree and later PhD, Kroto played lots of tennis and indulged his growing passion for art and design as art editor of a student magazine. Despite an interest in organic chemistry, he followed his fascination with quantum mechanics and spectroscopy to do his PhD on the spectroscopy of free radicals.
This led to a two-year stint as a post-doctoral fellow at the Canadian National Research Council facility in Ottawa, which he described as a ‘Mecca of spectroscopy’. Kroto relished the scientific freedom on offer, but also realised that his formal quantum theory training was slightly lacking compared to his physics-trained colleagues. However, this gave him the inkling that his chemistry nous might be useful in this sphere.
With this experience and a one-year spell at Bell Labs under his belt, Kroto headed back to the UK. Even then, Kroto had still entertained the possibility of moving into design, by pursuing a position with Buckminster Fuller – though on urban sub-structure design, and not geodesic domes. But before he had the chance to write to Fuller, an offer of a postdoc role at Sussex arrived; Kroto accepted, and the position quickly became a permanent lectureship.
The roots of fullerenes
Kroto followed his curiosity in the 1970s, investigating gas-phase free radicals and making compounds with double bonds between carbon and other main group elements – sulfur, phosphorus and silicon. ‘The work that gives me most intellectual satisfaction is not C60 – although it was a nice experiment, it was a serendipitous discovery,’ Kroto told Chemistry World in 2015. ‘I’m most fond of working out how to make a carbon–phosphorus molecule. People thought you couldn’t do it.’ Species with carbon–sulfur and boron–sulfur double bonds followed, but the silicon analogues proved elusive – which was to be a crucial catalyst to the discovery of C60.
During the 1970s, Kroto’s Sussex colleague David Walton was making linear chains of carbon atoms, polyynes, with alternating triple and single bonds. Kroto’s team then studied them on Sussex’s new microwave spectrometer. Around the same time, exotic molecular species, including HC3N, HC5N etc, were being identified in space by radio astronomy. Some were even unknown to chemists on Earth. It seemed as though long-chain carbon compounds were being found in space as quickly as the Sussex team could make and study them in the lab.
In 1985, Kroto was paying a return visit to Bob Curl at Rice University in Houston, US, when Curl told him that his colleague Rick Smalley had recently shown that SiC2 was triangular. This unexpected geometry helped explain why Kroto had had so much difficulty making carbon–silicon double bonds a decade earlier. Kroto and Curl visited Smalley’s lab and thus was a Nobel prize winning collaboration born.
Realising that Smalley’s cluster-beam apparatus could be made to mimic the conditions on the shell of a carbon-producing cool red giant star, Kroto proposed trying to produce carbon chains. Kroto and Curl also hoped that the experiment might hold the answer to astronomy’s mysterious diffuse interstellar bands. These spectroscopic anomalies had puzzled astronomers for decades – absorption bands in UV, visible and IR wavelengths found in the regions of space between stars, that corresponded to no known ions or molecules. It had recently been suggested that carbon chains could be the culprit.

The story of what happened next is well known. Together with students Jim Heath, Yuan Liu and Sean O’Brien, they quickly confirmed that the cluster beam environment could produce the C5–C9 carbon chains they had been hoping to see. They were puzzled, however, by a strong signal in the mass spectrum corresponding to a species with 60 carbon atoms.
They began to ponder what structure a stable cluster of 60 carbon atoms could be, mentally folding a sheet of carbon chicken wire into odd shapes. Kroto’s mind went back to the Montreal Expo. ‘That reminded me of Buckminster Fuller’s domes and I said we should get the book out, which Rick did,’ he said. After a day or two of speculation and model-building, the team settled on the now-famous structure.
A critical reception
As they were writing the paper describing their carbon soccer ball, the crucial question of the title arose. ‘I remember suggesting “C60: Buckminsterfullerene”, and I thought that would get people to think “What the hell is this?”’ Kroto said. ‘It turned out to be right, but it also turned out to be good because it brought other people to it.’
There were half a dozen papers by three of the major groups in the field who said we were wrong
The paper was submitted to Nature two days after their speculative structure had been finalised. ‘I think if it had gone to Science for refereeing they would have turned it down,’ Kroto said. ‘But it went to Nature and they were really nice referee reports: “I don’t know there’s much in this paper, but it’s a nice paper. A lot of people would be interested in this conjecture”,’ he recalled.
Unbeknownst to Kroto and the Rice team, they nearly missed out being first to the discovery. A team from Exxon in New Jersey, US, led by Andy Kaldor, had seen very similar results about two years earlier, but without grasping the significance. ‘Two other groups did the experiment. I don’t think [the discovery] would have lasted six months after us. Someone would have done it,’ Kroto said. So why did the Rice–Sussex team get there first? ‘The clue for me was what was going on was Buckminster Fuller’s dome, and that was through graphics, not science.’
There were many critics of the incredible-seeming structure, including Kaldor, and Kroto has called the period after the initial observation of C60 his ‘five years in the desert’. ‘There were half a dozen papers by three of the major groups in the field who said we were wrong,’ Kroto recalled. So the race was on to produce C60 in quantities that could be put in a bottle and held in the hand – or at least studied by more conventional characterisation methods. ‘I was absolutely convinced we were right and that one day we’d do it.’
Physics vs chemistry
Don Huffman, a physicist at the University of Arizona, US, had also been studying carbon as a route to understanding interstellar compounds, and had read the C60 paper in Nature. ‘After that excitement, everyone wanted to hold it in their hand. Smalley said: “I just want to see it”,’ Huffman recalls. Working with Wolfgang Krätschmer at the Max Planck Institute for Nuclear Physics in Heidelberg, Germany, Huffman was using carbon rod arc dicharge to produce soot, and infrared spectroscopy indicated that his samples contained reasonable amounts of C60. So the chemists at Sussex went head to head with the physicists in Arizona and Germany.
C60 had been synthesised in large quantities – and what’s worse, by physicists!
At Sussex, Jonathan Hare was tasked with synthesising C60, having originally joined Kroto’s group to study astrochemistry. ‘I didn’t know anything about C60 when I arrived. The idea was to get carbon rods and vaporise them. We did the experiments and were able to make the fullerene soot. 10% of that soot was C60 and we were able to extract it,’ he recalls. But Kroto and Hare’s joy at finally having C60 in a bottle in front of them quickly dissolved. They received Huffman’s paper to review for Nature just a few days later.
‘It threw Harry into despair!’ says Huffman, who had eventually drafted in the help of some chemists, and at their suggestion found C60 was soluble in benzene, giving them the production method that everyone wanted. ‘The first time I met Harry was a month or so later in Boston. He said “Oh my God, you’re the guy who’s made my life miserable!”’ Huffman says.
But Kroto is characteristically generous about Krätschmer and Huffman’s work. ‘I think their conjecture that what they had been looking at might be C60, and then going after the IR bands, then extracting it, I think that is one of the best, most brilliant pieces of chemistry of the 20th century. I really do.’ Kroto, Hare and colleagues were still able to publish the final piece of the C60 jigsaw, the single-line 13C NMR spectrum, which proved once and for all that C60 was a perfect sphere, with each carbon atom symmetrically equivalent. But Huffman and colleagues nearly beat them to this part too – it was only the limit on the number of figures imposed by the journal that stopped the Arizona team publishing an NMR spectrum. ‘I’m rather happy that Harry and his group were able to do that,’ Huffman says. ‘Because that, from the beginning, had been his greatest desire – that was the key that they were right on this crazy but beautiful structure of buckminsterfullerene.’
Gold rush

With a method now available to synthesis C60 in meaningful amounts, chemists across the world leapt at the chance to work with it. One of them was Fred Wudl, a physical organic chemist at the University of California in Santa Barbara, US. ‘C60 had been synthesised in large quantities – and what’s worse, it was done by physicists!’ he jokes. Working in organic electronics, C60 was a godsend. ‘It gave us a 3D pi-system, which is something we were all looking for, to expand the organic solid state. That time was so exciting, you could publish anything you wanted – even in Science or Nature. There was a tremendous upwelling of enthusiasm for spherical forms of carbon.’ Chemists like Wudl soon put C60 to work. ‘It was clearly a paradigm shift – we were responsible for an immediate jump in the power conversion efficiencies of organic solar cells. We made one that was between a hundred and a thousand times better than existed at the time.’
Now that C60 was firmly fixed in the textbooks as a third allotrope of carbon and being applied to everything from superconductors to solar cells, speculation mounted about the Nobel prize. ‘I knew it was possibly a contender, but whether it was 1%, 5%, 20%, 50%, I had no idea,’ Kroto said. ‘Of course, six years after the extraction, in 1996, we got the prize.’ The Nobel committee’s limit of only three winners meant Huffman and Krätschmer missed out. ‘I really wish they could have shared the prize. They deserve a share of it,’ he said.
Huffman is sanguine over the omission, however. ‘In competition like this in science, there are usually repercussions that are bitter. But the thing that I’m extremely proud of is that we came out of this as friends. I really treasure that.’
Reaching out
After winning the Nobel prize, Kroto used the increased profile to be a voice for science and scientists and to inspire young people to get a feel for science. ‘There are things I feel very strongly about, and I think science for me – a way that is true, what is experimentally proven – is more important to me than what people believe.’

Kroto was clear he saw this as his responsibility as a Nobel prize winner. ‘It’s nice to win it, but what’s the point? I’ve got a way to talk to young people about what I think is important and that’s why I do the outreach.’
Just one of example of his work is given by Diana Leitch, a chemist and trustee of the Catalyst Science and Discovery Centre in Widnes, UK. ‘In 2014, he did one of his buckyball events with 100 children, many of them from very deprived backgrounds from a school in special measures. And those children we so inspired by Harry that quite a few of them are coming along to an after-school science and engineering club with RSC funding – some of their parents even came!’ Demands on his time were intense, she recalled at the 2015 meeting. ‘We had to book him two years in advance. His wife Margaret needs to be mentioned, as she manages everything when he travels. They came to us from Sheffield, then drove to Sussex before flying to Moscow for three days. It’s his love of imparting science to children,’ Leitch says.
Hare ended up staying in Kroto’s group for 10 years, giving him a good insight into what make him tick. ‘Harry was an interesting mix of chemistry, spectroscopy and astronomy – he had all these interests. He loves solving puzzles, and everything was interesting when you have someone who’s interested and can show it you.’ Like Kroto, Hare is now a passionate science communicator, appearing in six series of the BBC TV series Rough Science.
Beyond personally appearing at conferences and events, Kroto set up the Vega Science Trust in 1995, making videos about science and scientists. Over 200 programmes were produced, many of them shown on BBC TV, and they are still available on the website, although the trust is no longer active. Where else can you see one Nobel prize winner (Kroto) interviewing a double Nobel prize winner (Fred Sanger)?
If Kroto was happy to inspire the next generation, he also had concerns over what sort of environment they will be working in as scientists. He doubted whether he would get funding for the experiments that found C60 nowadays. ‘You have to say “I want to do something important” to win a grant now. This wasn’t an important experiment to anyone except me – well, it wasn’t that important to me! It was interesting; a bit of a niggling thing.’
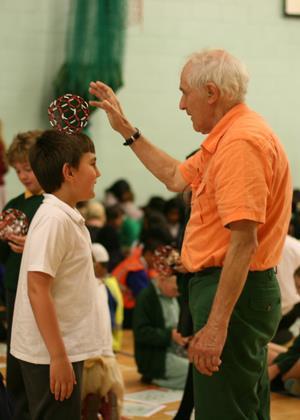
Kroto ignored research trends and bandwagons to follow his curiosity, leading him to discover C60 – which later became a bandwagon for many to follow, an irony that was not lost on him. But he was concerned that the recent funding climate is dulling scientists’ curiosity and creativity. ‘Our system is tending not to hone creativity and to kill off the adventurousness of people,’ he said. ‘I can understand that because it’s tough out there, and I think sometimes I was lucky that some of the things I thought of were easier than I expected. But I do seem to have an innate curiosity about things which other people don’t seem to have.’
Hare agrees with Kroto’s self-assessment. ‘I always think of Harry as like a detective – he’s interested if there’s something he doesn’t understand. Most of us, if we see something we don’t understand, we back away a bit. He gets excited meeting something he doesn’t understand.’ Huffman only has kind words about his former competitor: ‘He’s a visionary scientist. He’s a terrific spokesman for science. He was a great teacher of his grad students, but after C60, a marvellous teacher of the general non-scientific population – and children. He’s just a good person.’
Wudl recalls meeting Kroto at the first conference on fullerenes that Wudl organised in Santa Barbara. ‘You know right away you’re talking to a special person – a special character. And I haven’t changed my mind since!’
The final piece of the puzzle

But what about those mysterious astronomical anomalies, the diffuse interstellar bands, that Kroto, Curl and colleagues had hoped to unravel – was C60 really the culprit? On the first day of the 2015 symposium celebrating 30 years of fullerenes, John Maier of the University of Basel in Switzerland published a paper in Nature that finally proved that C60+ was indeed one of the culprits behind the diffuse interstellar bands. Maier, a friend of Kroto’s since the 1970s, had finally proved what Kroto, Curl and Smalley set out to do in 1985. The timing couldn’t have been better, and Kroto, attending the meeting along with Maier, was overjoyed. ‘It’s the paper of the year, and the proof that C60+ is all over the galaxy is amazing. Who would have thought there were footballs all over the galaxy?’
Kroto had been hoping Maier would do the experiment for a long time. ‘Immediately after the discovery of C60, people started looking for it in space and the signatures weren’t found in the diffuse clouds,’ he said. ‘It took 20 years to develop the techniques to measure the electronic structure of C60+ at 6K, under conditions like in space, to be able to make a direct comparison.’
Maier’s team got the results a few months prior to the 2015 meeting, but kept it a secret until a few weeks before. ‘When I told Harry he was very emotional. He said “John, you’ve made me a very happy man!”’
Tony Cheetham of the University of Cambridge, UK, was a long-time friend of Kroto, and helped to organise the 2015 meeting with Ale Palermo of the RSC after learning that Kroto was suffering from motor neurone disease in the summer of 2014. For Cheetham, the meeting had three purposes: to celebrate 30 years of fullerenes and Kroto’s 75th birthday in the autumn of 2014, and because he was concerned that Kroto’s health might fade. ‘I wanted to do something while he was still fit and that he could enjoy, which obviously he did,’ says Cheetham.
In spite of the diagnosis, Kroto continued to travel and appear at events in 2015, according to Cheetham, including speaking at a UK–South Korea workshop organised by the Royal Society and the Institute for Basic Science in September 2015. But by March 2016, Kroto was in hospital and passed away on Saturday 30 April.
With reporting by Philip Robinson
Updated 19 May 2016 with comments from Tony Cheetham












No comments yet