From wooden models to thousands and thousands of structures, Julia Robinson tells the story of how Richard Robson, Susumu Kitagawa and Omar Yaghi won the 2025 Nobel prize in chemistry
- Origins and development of MOFs: Metal–organic frameworks (MOFs) evolved from Richard Robson’s 1970s teaching models using wooden balls and rods, leading to the creation of porous crystalline structures. His early work laid the foundation for the field, despite initial instability in the materials.
- Stabilisation and functionalisation: Susumu Kitagawa advanced MOFs by developing more stable and functional frameworks using cobalt(ii) ions and neutral ligands, overcoming skepticism and funding challenges. He introduced the concept of MOF generations, including flexible and multifunctional designs.
- Expansion and reticular chemistry: Omar Yaghi coined the term ‘metal–organic framework’ and revolutionised the field with MOF-5, demonstrating permanent porosity and gas adsorption. He introduced reticular chemistry, enabling the design of MOFs with tailored properties for specific applications.
- Applications and impact: MOFs might be used in gas storage, environmental remediation, catalysis, medicine and more. Despite challenges in scalability and synthesis, their versatility has led to commercialisation and global recognition, culminating in the 2025 Nobel prize in chemistry for Robson, Kitagawa and Yaghi.
This summary was generated by AI and checked by a human editor
With their seemingly infinite potential applications, metal–organic frameworks (MOFs) are a vibrant international field of research. But just a few decades ago, these highly porous materials, in which metal ions are linked together by organic molecules in a regular repeating pattern to create a 3D network, were but a fleeting concept in a university lecture hall.
The expansion of the field since then can only be described as an explosion. In the early 2000s, there were just a handful of research groups dedicated to MOFs and a few dozen papers published each year but now the rate of publishing has increased exponentially – with almost 10,000 papers published in 2024 and over 100,000 MOF structures recorded to date.
The recognition of Richard Robson of the University of Melbourne in Australia, Susumu Kitagawa of Kyoto University in Japan and Omar Yaghi of the University of California, Berkeley in the US in this years’ chemistry Nobel prize for the development of MOFs will have come as little surprise to many in the field.
‘We were so excited, over the moon, and we started clapping,’ says Kyriaki Koupepidou, a postdoc in Kitagawa’s lab. ‘It was such an unreal experience – we had a big screen, and they pulled up the picture of the three pioneers, and we kept that on the screen as inspiration for our experiments.’
However, the endless applications for these structures – and the speed at which the field developed – is something few could have predicted in those early years.
‘One of the things that I will treasure in my retirement is just being lucky to see a whole new field of chemistry develop right from the start and from a front row seat,’ says Stuart Batten, a chemist at Monash University in Melbourne, Australia, who was the first student in Robson’s group to work on MOFs.
‘I can remember we would go down to the library and look at the journals, which in Australia were about three months behind the rest of the world because they had to be posted, and every few months you’d go “Oh, someone else has done one. We’ve got competition.” And now, you literally just can’t keep up,’ he says. ‘Seeing that transition from our group being the only ones talking about it at conferences, to now whole conferences [and] whole journals devoted to the field is still a little bit surreal.’
Inspired by wooden rods and balls
There are some indications that coordination-type polymers and frameworks go back as far as the 1800s, but Robson’s seminal work stems from the 1970s, when he was teaching undergraduate students at the University of Melbourne in Australia. In 1974, about eight years into his time teaching chemistry at the university, Robson got a new boss who was keen to come up with new ideas to brighten up their teaching. He tasked Robson with producing models from wooden balls and rods to enable first year undergraduates to explore chemical structures.

Using log tables, Robson had to calculate the angles at which the on-campus workshop would have to drill holes in the wooden balls to represent the atoms in the structure. It was when he was assembling the models for the first time that the thought arose to Robson of what would happen if he linked together different types of molecules, rather than individual atoms – would it be possible to design new types of molecular constructions?
He initially forgot the idea but then, every year, as these wooden models were brought out of storage the thought would come back to him. Eventually, after 10 years, he decided to put the idea into practice.
Inspired by the structure of diamond, the first compound Robson created was a similar structure based on positively charged copper ions. The copper ions were combined with a molecule with four arms – a tetracyanotetraphenylmethane – each with a nitrile group at the end that was attracted to the copper ions. The result was a large molecular construction with a regular crystalline structure like diamond. However, unlike diamond, it contained a number of large cavities filled with randomly distributed and freely moving solvent molecules and counter ions.
‘I think even to his own surprise, it actually worked … he expected to just get amorphous rubbish in the bottom of his reactions, instead of these beautiful little crystals,’ says Batten.
The birth of coordination polymers
In exploring these ideas, Robson worked closely with his colleague and close friend Bernard Hoskins, an x-ray crystallographer. Previously Robson had been quite isolated in his work, but he says it was through his collaborations with Hoskins, and later, the inorganic chemist Brendan Abrahams, that ‘the whole thing became viable’.
‘In the late 1980s, I was able to get compounds in crystalline form and Bernard was able to determine the structure and things began to develop from there … we began to get students … and then it expanded from there,’ Robson explained in a door-step interview at the University of Melbourne the day after the Nobel prize was announced.
He published his work in 1989 in the Journal of the American Chemical Society, in which he referred to the structures as ‘coordination polymers’ – a term he still prefers over MOFs. In other interviews he has said he realised ‘almost immediately’ that this was going to be a big field of science.
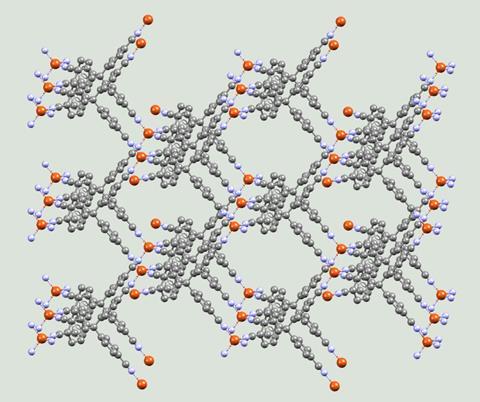
‘It was very exciting because all those structures were new,’ says Batten. ‘No one had done anything like that before. Nowadays, people are used to seeing the beautiful symmetry and connectivity and architecture of MOFs, but in those days, it was a complete surprise, every one that turned up.’
Robson went on to create several new types of molecular constructions with cavities filled with various substances, and showed they could perform ion exchange. He also predicted several important features of these frameworks: the possibility of integrating catalytic sites within the frameworks and the generation of efficient heterogenous catalysts, for example.
Deanna D’Alessandro, a chemical and biomolecular engineer at the University of Sydney, says that Robson’s work was ‘a very important discovery’ for the Australian chemistry community.
‘We have this interesting lineage in Australia, where coordination chemistry in particular, and inorganic chemistry is a very strong discipline of chemistry – many of us went overseas about the same time, and then we came back to Australia. [Now] we’re mid-career researchers who were in the cohort who saw that great history and wanted to carry that on for Australia.’
But a significant problem with Robson’s frameworks is that many of them were unstable and collapsed easily; the next part of the story, leading to more stable structures, took place in Japan.
Kitagawa’s quest for stability
Kitagawa had been in good company during his PhD in the quantum chemistry lab at Kyoto University – his academic ‘grandfather’ was Kenichi Fukui who shared the 1981 Nobel prize in chemistry for his theories on the course of chemical reactions, while senior to him in the same laboratory was Akiro Yoshino, who shared the 2019 prize for his work on lithium-ion batteries.

During his academic career Kitagawa had always had a lot of freedom to explore his research, but it was when he moved onto a private university – Kindai University – that his boss was keen that they start working on copper(i) chemistry, although many of his peers couldn’t understand why.
‘[With copper(i)] the d-shell is fully occupied, [there’s] no d-d transition, no colour, no magnetism,’ explains Kitagawa. ‘So most of the coordination chemistry society said “Why you are doing such boring copper(i) chemistry?”’
However, he likens the chemistry of copper(i) to a ‘gold mine’. ‘Copper(i) has a d10 shell that has a spherical charge distribution … that means [it is] easy to crystallise, to show an extended structure. In the case of iron [or] cobalt, the electron distribution is isotropic so it is very difficult to show such a beautiful structure.’
He explained how using copper(i) they obtained some ‘very beautiful structures’ but that, at times, it was challenging because they often had to borrow the crystallography equipment. They persisted, however, and one day they observed a porous structure.
‘My student said “Professor, this has many pores, and in the pores, the organic molecules are accommodated,”’ Kitagawa remembers. ‘That was my inspiration, completely. That’s the point. I switched my target [to porous structures] – the turning point.’
They published a paper in 1992 to show their first molecular construction – a two-dimensional material based on copper(i) coordinated with pyrazine and acetonitrile, containing cavities with loosely bound acetone molecules.
‘Many, many people told me “This structure looks like a zeolite, but you synthesised this using organic molecules. This should not be stable,”’ says Kitagawa. And it was true: the structure used neutral ligands meaning the bonding energy was weak – around 100 kilojoules per mole.
So Kitagawa set out with a strategy of building a structure that still had neutral linkers but that had stronger bonds and was therefore more stable, while also maintaining the all-important porous structure. However, he was somewhat hindered along the way – research funders could not see the point to his work and many of his grant applications were rejected.
‘In Japan, we have the fundamental research fund – it is competitive, but the success rate is 20–25%. But the total fund is not so large, so we tried to get the larger funds, because for porous materials chemistry, we need a lot of instruments,’ he says. ‘Ultimately, I had an interview, but the interviewer said “This is unstable, we cannot believe [in it].” So I was disappointed.’
But he was not put off. Kitagawa was inspired by a quote from the famous French chemist and microbiologist Louis Pasteur ‘chance favours only the prepared mind’ and he ultimately believed that this was an area of chemistry that was only set to expand.
A vision of frameworks
For five years he persisted through an endless process of trial and error until finally, they had a breakthrough – creating a framework that was both stable and had a function – when they decided to switch the copper(i) to another metal ion – cobalt(ii) – along with a neutral organic ligand molecule, 4,4′-bipyridine.
‘Using a neutral molecule means we obtain the porous structure – we can get the cavities,’ Kitagawa explains. ‘So, we succeeded. Also, instead of the Cu+ we used Co2+ and this shows the bonding energy to be more than 200kJ per mol – so a stronger bond.’
This work was published in a 1997 paper in which his team also demonstrated successful gas adsorption of methane, nitrogen and oxygen at room temperature. The cavities of the cobalt(ii) structure were initially filled with water, but the framework could be shown to adsorb and release the gas substances when in a dry state.
![Crystal structure of Kitagawa's 1997 framework material {[M2(4, 4′-bpy)3(NO3)4]·xH2O}n (M = Co, Ni, Zn)](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/480xany/4/5/1/543451_rocyaj_2copy_604005.jpg)
To maintain momentum, Kitagawa knew that he had to convince funders of what made metal–organic frameworks unique. In 1998, he set out his vision in the Bulletin of the Chemical Society of Japan, laying out the notion of different ‘generations’ of frameworks – from the structurally beautiful, but unstable, to the practically robust and useful.
It was at this point that he predicted that MOFs should have a level of softness and flexibility – a third generation of MOFs – and while no one was absolutely against this, they wanted to see an example. So Kitagawa set out to develop a flexible MOF.
‘In 2002, we observed the first one – [with] a very beautiful hysteretic adsorption that is very close to the haemoglobin oxygen adsorption … all the people believed this – it was easy to show the public,’ he says.
Kitagawa later went on to develop the idea of a fourth generation of MOFs which referred to a comprehensive design of microstructures leading to complex functionality.
Yaghi and MOF-5
However, Kitagawa was not the only one who had been working hard to develop MOFs during this time. Starting out as a research group leader at Arizona State University, US, in the early 1990s, Yaghi was keen to find more controlled ways to create materials, using rational design to connect different chemical constituents to make large crystals.
The work was challenging, but a few years later his team started combining metal ions with organic molecules. In 1995, Yaghi published the structure of two different 2D materials that were like nets and held together by copper or cobalt. They demonstrated that the cobalt structures had channels in which aromatic guest molecules could selectively bind and were so stable they could be heated to 350°C without collapsing, even after removal of the guest molecules. It was in this paper that he first coined the term ‘metal–organic framework’.

However, it was in 1999 that Yaghi established the next milestone in the development of MOFs when he presented the synthesis of MOF-5, an exceptionally spacious and stable molecular construction that, even when empty, could be heated to 300°C.
‘A gram of a MOF, which is no larger than a sugar cube, encompasses a space as large as a football field,’ explained Yaghi in a press conference organised by the University of California, Berkeley, following the Nobel prize announcement on 8 October 2025. ‘So you can imagine how powerful these materials are in terms of compacting gases without having to use lots of energy, such as high pressure or low temperature.’
‘The moment that the whole field changed was the MOF-5 paper that Omar published, where he showed that you could remove guests from the pores of these materials, and they would remain permanently porous and take up gas,’ says Christian Doonan, a professor at the University of Adelaide in Australia, who worked as a postdoc in Yaghi’s lab between 2007 and 2010.
‘I remember Omar telling me the story about how after publishing that paper, he got a call from Ullrich Müller at BASF, who basically said “Is this real?” And Omar said “Yes”. And he said, “Well, we should meet.” That was the start of an enduring relationship between Omar and BASF with respect to metal–organic framework chemistry.’
The reticular revolution
Yaghi showed that it was possible to modify and change MOFs to give them different properties. To highlight this, he produced 16 variants of MOF-5, with cavities that were both larger and smaller than those in the original material. It was in a 2003 review article that he defined the term ‘reticular synthesis’ (or reticular chemistry) as ‘the process of assembling judiciously designed rigid molecular building blocks into predetermined ordered structures (networks), which are held together by strong bonding’. He further expanded on this idea with the term ‘isoreticular’.
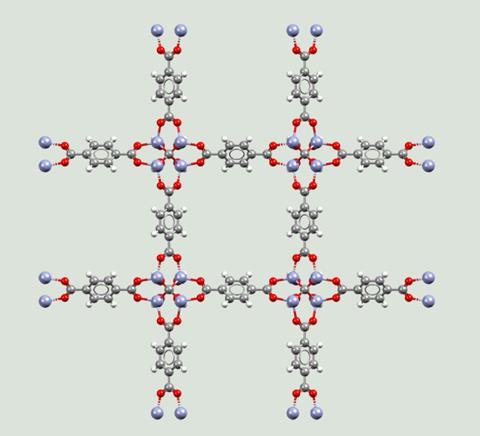
‘Isoreticular design [is] where you take a known structure and then by changing the building blocks – for example, the length of the building blocks – you can preserve the topology while changing the functionalities,’ explains Petra Ágota Szilágyi, an expert in porous materials and catalysis at the University of Oslo in Norway. ‘By enlarging or lengthening the linkers, you can make the pores bigger.’
This is helpful when adapting MOFs for specific applications.
‘For gas capture, you want to adjust these properties really judiciously,’ Szilagyi adds. ‘So you want to have the pore sizes of a particular diameter. Also, you want to have potentially particular functional groups in the linkers or on the nodes which will interact at a particular strength with the gas you want to capture. This is really the philosophy, that you can take these generic structures and you can, by altering or varying components of them, fine tune their properties.’
MOFs’ killer apps
It was after these breakthroughs that MOFs took off in a big way. When Doonan joined Yaghi’s group a few years later, he says the trajectory of MOFs was ‘almost vertical’.
‘It was at the point where the chemistry was reasonably well understood about how to make these materials and how to study them. Then I started in the period of “As chemists, what can we do with these materials?”’ Doonan says. ‘That was an incredible time to be in the laboratory – other people had done the hard work in breaking new ground, and then I managed to join the lab when the field was accepted and really taking off.’
The large surface area, designability and ease of fabrication of MOFs (or coordination polymers) have provided chemists with a valuable ‘toolkit’, says D’Alessandro.
‘You’re a bit like an architect, and you can decide “I want this functionality, I want this shape, this size, this colour, I want that over there.” And you can do that in a MOF. You can have different properties. Over the years, we’ve seen the usefulness [and] practicality of MOFs evolve, and their ability to be scaled up with a view towards practical, real-world applications.’
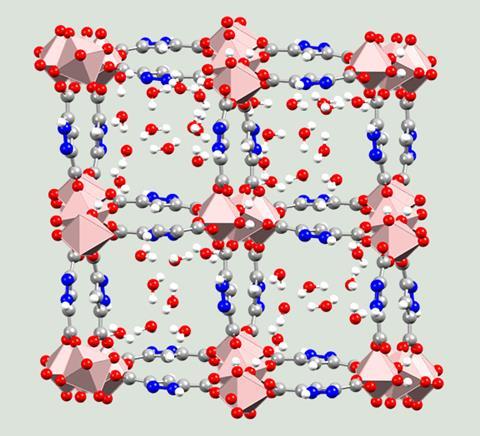
The development of new MOFs has focused around three key functions – storage, separation and conversion – and the potential applications from these are almost endless.
‘These frameworks encompass space within which one can trap gases such as carbon dioxide or hydrogen,’ Yaghi said during the 8 October press conference. ’They could even be designed to seek out contaminants in water like PFAS or organic contaminants from water or contaminants from the atmosphere. The amazing thing is that you can also modify their pores so that you can catalyse reactions and some applications where we take poisonous molecules and make them to be harmless molecules through passing them through the pores of these materials. The power of MOFs is that they open new avenues of applications that other materials could not do.’
In addition to gas storage, applications include biosensors, batteries and fuel cell technology, separation science, synthesis and catalysis, water harvesting, and capture and destruction of harmful agents. There are also several medical applications including drug delivery, biobanking and diagnostics and even some potential uses in defence.
Challenges and opportunities
‘If there are going to be game-changing solutions to the world’s problems, there’s a very good chance that a MOF will be at the heart of it,’ says Mike Zaworotko, a crystal engineer at the University of Limerick in Ireland. ‘MOFs offer a limitless range of opportunities and properties. It’s focusing on the immediate tasks and the right MOF for the right application – that’s now the challenge.’
Russell Morris, a chemist at the University of St Andrews, in Scotland, says the environmental applications of the MOFs are the ones that are most developed. ‘Carbon capture, hydrogen storage, methane storage, water harvesting, all those sorts of things – those are the ones that really get a lot of people interested.’
However, he says, ‘The world is our oyster. There’s so many different possibilities, and everybody’s coming up with different ideas.’
Although many MOFs that have been created have only been used on a lab scale, many companies are now investing in their commercialisation. In 2016, for example, NuMat Technologies in the US launched ION-X, a compressed gas cylinder the uses the storage function of MOFs to enable the safe transportation of hazardous gases. In 2023, BASF became the first company to successfully produce MOFs on a commercial scale for carbon capture for the Canadian technology provider Svante Technologies.
But, as versatile as they are, MOFs are not without their challenges – not all MOFs can be scaled up easily while also maintaining their properties, and there are also considerations to be made around cost and potential environmental toxicity. ‘A big problem is also their synthesis – often it takes place under harsh conditions,’ says Szilagyi. ‘But there have been beautiful examples in which they were able to use green chemical concepts … more sustainable precursors and reagents, but also making the conditions milder.’
Despite the challenges, it’s clear that this year’s Nobel prize has gone to the fathers of a field that has already had a significant impact on the world. ‘We’re seeing the translation, and we’re seeing the commercialisation,’ says D’Alessandro. ‘Their usefulness and their practical application has become apparent … it’s clear that they have made a really big imprint on the world.’
‘This is sort of the end of the beginning,’ says Zaworotko. ‘The design and recognition of the field is there. There’s no upper limit to how many MOFs you can make.’
Julia Robinson is a science correspondent for Chemistry World
















No comments yet