Molecular switch enables non-invasive control of chirality
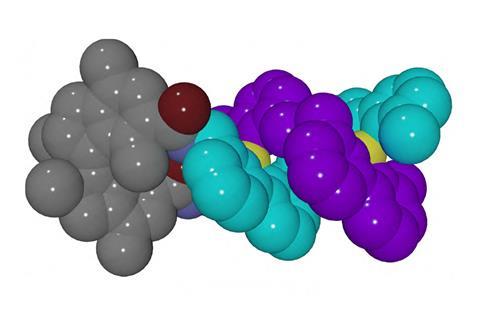
Researchers in the Netherlands have used a light-sensitive molecular motor to control the chirality of a double-stranded ‘helicate’. The motor mimics the way that nature employs certain enzymes to unwind helical structures such as DNA.
Helicates are polymetallic complexes in which two organic ligands weave around metal centres and each other to form a double helix – similar to the structure famously observed in DNA. They were first discovered in 1987 by Jean-Marie Lehn, who shared a Nobel prize that same year with Donald Cram and Charles Pederson for pioneering work in the field of supramolecular chemistry.
Almost 30 years since the original research on helicates, a team of scientists led by another Nobel laureate, Ben Feringa of Groningen University, has adapted Lehn’s system to make a new type of molecular machine.
The team can dictate the direction two ligands weave around each other in dinuclear copper complexes, by capping the system with a molecular motor – one of the nanoscale machines that saw Feringa awarded with the 2016 Nobel prize, alongside Fraser Stoddart and Jean-Pierre Sauvage. ‘The idea was to use our molecular motor as a multi-state switch,’ explains Feringa. ‘Because it goes through a rotary cycle, we can address different states which have different chirality.’
The rotor is attached to the ends of two oligobipyridine ligands, which eventually form the strands of the helix. When copper is added, the system self-assembles into a helical structure in a favoured chiral configuration. When this system is treated with light and heat, the motor rotates, causing the helix to unravel and eventually reform as the opposite enantiomer. The chirality of helical molecular systems, including certain antibiotics and catalysts, is often crucial to the way that they carry out their biological or chemical functions.
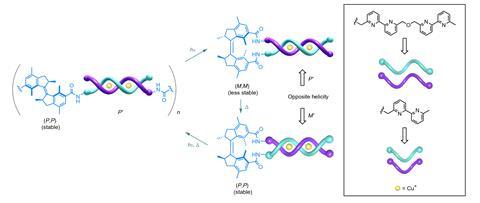
Markus Albrecht, a supramolecular chemist at RWTH Aachen University, Germany, praises the ‘unique example of stereochemical switching of helicates’, adding that the research could pave the way for new ‘systems for control of chemical reactivity, molecular recognition and even with special mechanical properties’.
Bert Meijer, who also works on supramolecular systems at Eindhoven University of Technology, Netherlands, is similarly impressed with the new rotary motor scaffold. Describing the work as ‘a beautiful illustration of why the 2016 Nobel prize in chemistry was given for the design and synthesis of molecular machines’, he adds that ‘in every new contribution of the [Feringa] group, they come closer to mimicking natural molecular machines with very innovative and original designs’.
Looking to the future, Feringa says that the system could be used for the design of new selective catalysts. ‘Metal complexes that have a certain chirality can act as chiral catalysts,’ he says. ‘By using an external stimulus we might be able to change the configuration of the catalyst and as a result the outcome of the product.’
References
D Zhao et al, Nat. Chem., 2016, DOI: 10.1038/nchem.2668













No comments yet