Learning to mimic natural photosynthesis on an industrial scale might open the door to a fossil fuel-free future. Nina Notman investigates
We have created a global climate emergency and our reliance on fossil fuels is largely to blame. To save our planet, our global love affair with fossil fuels must end – fast.
We can now produce electricity without emitting carbon dioxide, and carbon-neutral electricity production methods are ever more common place. But practical and cost issues mean that coal and natural gas continue to dominate in most parts of the world. Renewable energy, such as that from wind turbines, solar panels and hydroelectric power stations, for example, has problems with intermittency, storage and portability. Nuclear power is another low-carbon method, but suffers from a negative public perception and toxic waste logistics.
Electricity production is actually the easy bit. It’s much harder to heat homes, power planes, trains and automobiles without using chemicals derived from fossil fuels, not to mention producing the multitude of compounds and materials that our modern world is dependent upon. ‘What we’ve done so far, is move towards decarbonising electrical power generation and increased energy efficiency. That’s fantastic, of course, but they are the low hanging fruit,’ explains James Durrant, a photochemist at Imperial College London and Swansea University in the UK. ‘At some point we will have to go beyond that and wean the whole of the chemical industry off of fossil fuel-derived materials.’
Chemical fuels produced by artificial photosynthesis could replace those from fossil fuels
Biofuels are a step in the right direction. But carbon-neutral alternatives with properties indistinguishable to those from fossil fuels are not yet available. One technology is, however, edging ever closer to making these a reality: artificial photosynthesis. Chemical fuels produced by artificial photosynthesis are ‘really quite analogous’ with those from fossil fuels, explains Erwin Reisner, a renewable energy researcher at the University of Cambridge in the UK. This means that – once produced – they could slip seamlessly into existing infrastructure. ‘In principle, we should be able to replace pretty much everything that is done by fossil fuels with artificial photosynthesis,’ says Reisner. ‘This is a dream scenario, but the underpinning chemistry is there.’
Harvesting energy from the sun

The energy in fossil fuels originated, millions of years ago, from the sun. Plants use solar energy to split water into oxygen gas and hydrogen stored as the coenzyme NADPH (nicotinamide adenine dinucleotide phosphate hydrogen). The hydrogen then reacts with carbon dioxide captured from the air, producing storable chemical energy in the form of carbohydrates. Over millions of years, the heat and pressure of the Earth’s crust has turned these photosynthesis products into fossil fuels.
‘Artificial photosynthesis is inspired by natural photosynthesis,’ Reisner explains. ‘We are trying to harvest sunlight and use that energy to make chemical fuels from abundant feedstocks such as water and carbon dioxide.’ But the snag is we need to do so much faster and with greater efficiency. Plants only convert about 1% of the solar energy they receive into chemical energy.
The idea has been around for over 100 years but the field didn’t really take off until the 1970s, when the first oil crisis caused oil prices to rocket, Reisner says. ‘When the oil price dropped again, activities slowed down very significantly. Then, around 10–15 years ago, the field started to grow very quickly and has now gained substantial momentum.’
‘Today, it’s a very diverse field,’ agrees Durrant. ‘There are almost as many materials studied and device concepts as there are people working in the field.’ No devices yet operate on an industrial scale, but those that are closest are all sequential technologies.
Ploughing ahead
A small number of sequential artificial photosynthesis pilots are already in operation. These all decouple the sunlight capture from the rest of the process, with some pilots using integrated solar cells and others renewable energy from the grid to power water splitting via electrolysis. Critics point out, however, that many of these merely delay the inevitable: the carbon dioxide is from fossil fuels and still released into the atmosphere at the end of the process.
The George Olah Renewable Methanol Plant, for example, which opened near Grindavik in Iceland in 2012, uses renewable electricity from the grid (either hydroelectric or geothermal). The hydrogen produced is converted to methanol in a catalytic reaction with carbon dioxide, captured from the flue gas of a neighbouring geothermal power plant. According to its operator, Carbon Recycling International, the methanol plant now recycles 5500 tonnes of carbon dioxide that would otherwise have been released into the atmosphere.
Making carbon–carbon bonds from carbon dioxide looks very simple but it is a tremendous technical challenge
The Carbon2Chem pilot plant in Duisburg, owned by German steel manufacturer ThyssenKrupp, launched in 2018. Its electrolysis step is powered by surplus renewable grid energy. The resulting hydrogen is combined with carbon dioxide (separated out from a nearby steel mill’s waste gases) to produce methanol, again with the help of a metallic catalyst (still under development). ThyssenKrupp says that its technology will take around 15 years to reach an industrial scale. Ammonia is also produced at the plant, important because the Haber–Bosch process currently produces approximately 1% of global greenhouse gas emissions. Polymers and higher alcohols are longer-term goals.
‘Making carbon–carbon bonds from carbon dioxide looks very simple on first sight but it is a tremendous technical challenge,’ explains Matthias Beller, director of the Leibniz Institute for Catalysis and lead of a recent joint position paper by the German academies of science on artificial photosynthesis. It is also a reaction that biology currently performs better than chemistry: enzymes display greater selectivity and greater efficiency at turning carbon dioxide into products containing more than one carbon atom, compared to their state-of-the-art chemical counterparts.
Industrial conglomerate Siemens and speciality chemical company Evonik are harnessing the power of enzymes in their joint research project, Rheticus. An artificial photosynthesis pilot plant, scheduled to go on stream by 2020, is currently being built at the Evonik facility in Marl, Germany. It will use solar panels, followed by a unique electrolysis step. Water is split and carbon dioxide reduced simultaneously – producing syngas, a mixture of carbon monoxide, hydrogen and carbon dioxide. They envisage the carbon dioxide coming from sources such as biogas plants, explains Thomas Haas, vice-president of science and technology at Evonik Creavis.
The syngas is then fed to bacteria during the fuel-formation stage. ‘The second unit of the system is a biotech reactor also called a fermentor,’ says Haas. ‘It’s like beer production, where the microorganisms produce ethanol. Our microorganisms produce, for instance, butanol and hexanol instead.’ The compounds produced depend on both bacteria type and the environment they are kept in. ‘The organisms need salt and vitamins, and they can change the product depending on the additives available,’ Haas explains. ‘We don’t do genetic engineering at the moment. Nevertheless, if we talk about the future – 10–20 years ahead – this offers a huge potential to produce many more different chemicals that we do at the moment.’
The first Rheticus pilot will operate at a scale of producing kilograms per hour. ‘We will then go stepwise,’ says Haas. ‘In five years, we try to achieve speciality chemical scale: 10,000 tonnes per year.’
Putting a foot on the accelerator
To recap, the sequential technologies that we are starting to see built on the pilot-plant scale have three distinct steps – energy capture from sunlight, using this energy to split water into hydrogen, and then using the hydrogen to reduce carbon dioxide to hydrocarbon-based fuels. Researchers are keen to explore the potential efficiency, time and cost opportunities available if any of these stages can be done simultaneously.
Electrocatalysis, able to split water and produce fuel in a single step, has potential here. ‘There are two ways of doing artificial photosynthesis, you can either reduce protons to hydrogen and then use that hydrogen to reduce carbon dioxide, or alternatively use the electrons to directly reduce carbon dioxide,’ explains Durrant. The ultimate goal is integrated devices, where sunlight is captured, water split and fuel produced in a single step. The related field of photoelectrocatalysis is the likely contender to enable this.
The problem is that carbon dioxide reduction is quite a lot harder than proton reduction
‘Electrocatalytic conversion is an electrochemical process so there’s two half reactions,’ says Phil De Luna, programme director at National Research Council Canada (NRC) in Toronto. ‘On the anode side, you’re typically oxidising water and turning it into oxygen and protons. And on the cathode side, you’re using those protons to convert carbon dioxide into a reduced and higher value product,’ he explains.
‘The problem there is that the catalysis of carbon dioxide reduction is quite a lot harder than the catalysis of proton reduction,’ adds Durrant. ‘We currently have some materials and devices which are efficient, we have some materials and devices which are cheap, and we have some materials and devices which are stable, but we don’t have any materials which are all three.’
Another ongoing challenge, according to De Luna, is product separation. Even if you have a really selective catalyst ‘when you’re converting carbon dioxide, some of it won’t get converted’, he explains. Cleaning up the product can be very energy intensive.
Commercially viable electrocatalysts and photoelectrocatalysts for artificial photosynthesis is the focus of many research groups around the world, including De Luna’s newly-formed NRC team and the research group he graduated from in early 2019 – that of Ted Sargent at the University of Toronto.
At the coal face
The Sargent group, in collaboration with Dave Sinton’s group at the same university, is currently building an electrocatalysis-based artificial photosynthesis pilot plant, as part of the $20 million (£15 million) NRG Cosia Carbon XPrize competition; Cosia, ironically, is Canada’s Oil Sands Innovation Alliance. ‘The Carbon XPrize is a global competition to capture and convert the most carbon dioxide into the most valuable product,’ says De Luna. ‘In the semi-final round we scaled up our process to a few kilograms per day. This was an order of magnitude scale up in less than a year. Now the team is building a pilot plant in Alberta for the final round of the competition.’ For the pilot, the team will convert carbon dioxide being emitted from a natural gas power plant.
We can play with the catalysts to change products to what we want
‘Our team is one of the 10 finalists of this competition,’ explains Fengwang Li, a postdoc in Sargent’s group. The team is using flow-cell reactors. The prototype is about 1cm2 and Li says they are currently scaling up stepwise to 1000cm2. Ultimately, stacks of these larger flow cells will be run in parallel. ‘In the flow cell device, we have a lot of interfaces which allow the carbon dioxide, the catalysts and the electrolytes to meet together with a lot of reactive area so that we can convert a lot of carbon dioxide in a very short time. Our target for each cell stack is to convert a few hundred kilograms of carbon dioxide per day,’ he says.
In the previous round, the team demonstrated conversion of carbon dioxide into carbon monoxide. For the final, they are aiming to produce ethylene, a much more versatile chemical feedstock. ‘The product we achieve is catalyst-dependent not device-dependent. If you use silver catalyst, you can produce carbon monoxide, and if you change the silver into copper, you can directly convert the carbon dioxide to ethylene,’ says Li. ‘Once we have the fully optimised device, then we can play with the catalysts to change products to what we want.’
Coming up for air
The catalysts that control product selectivity are located at the cathode. But there is a second catalyst in these electrochemical devices – on the anode. This is the site of oxygen generation. ‘Much of the energy consumption come from the oxygen evolution’ at the anode, explains Li.
We’re now trying to transfer all that we’ve learnt with ruthenium to iron or copper
Energy-efficient water-oxidation catalysts are therefore the focus of many research groups worldwide, including that of Antoni Llobet at the Institute of Chemical Research of Catalonia in Spain. Good ruthenium-based catalysts have now been developed in his group, but this metal is too expensive for commercial use, he explains. ‘We’re now trying to transfer all the information that we’ve learnt with ruthenium to metals such as iron or copper. The device that you would end up making would then be much cheaper.’
Llobet is also tackling the design of photoelectrochemical water splitting cells. He is replacing the electrode at both the anode and cathode with light-absorbent materials. These create charge when illuminated, driving the electrochemical catalysts attached to their surfaces.
Lighting up the future
Daniel Nocera at Harvard University in the US is taking a unique approach to producing fuels from artificial photosynthesis. In 2011, Nocera revealed his artificial leaf – a multilayered catalytic water-splitting device. This leaf is comprised of layers of multiple cheap catalysts including one to capture solar energy, and separate oxygen- and hydrogen-forming catalysts. ‘The device is made from entirely earth-abundant materials with no wires,’ Nocera explains. ‘We put it into a glass of water and shine light on it to split the water to hydrogen and oxygen.’
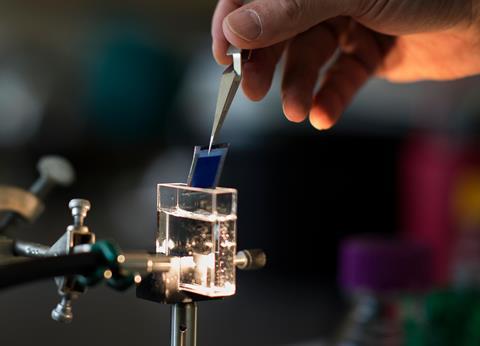
Having cracked hydrogen production, the Nocera group turned its attention to hydrocarbon-fuel formation. The resulting device, which uses genetically engineered bacteria that take in carbon dioxide from the air, is coined the bionic leaf. ‘The bionic leaf takes the hydrogen from the artificial leaf, and combines it with carbon dioxide from air to make biomass or fuels,’ says Nocera. ‘The bugs are engineered to eat hydrogen and it into cellular energy,’ he adds. They then convert the carbon dioxide to other chemicals using carbonic anhydrase enzymes. This type of technology is seen by some as the final goal for artificial photosynthesis as it doesn’t rely on a waste carbon dioxide source. And ultimately, we should be aiming for a world where energy production and industry do not produce carbon dioxide waste at all.
So far, the bugs in the bionic leaf can produce hydrocarbon fuels ranging from the C2 alcohol ethanol up to C14 hydrocarbons. The same device is also being used to make fertiliser, by fixing nitrogen from the air.
Nocera also has an eye on robustness – using self-healing catalysts in the artificial leaf that can even work with dirty water and bugs that self-replicate in the bionic leaf. He foresees the device, therefore, being used in remote, off-grid locations. ‘We’re currently building pilot-scale 1000L reactors at the Institute of Chemical Technology in India,’ he says.
Stronger together
Kazunari Domen at the University of Tokyo and Shinshu University, Japan, is also taking an unusual approach to artificial photosynthesis – developing photocatalytic sheets able to split water. ‘These are interesting because they show quite promising efficiencies and, more importantly, they are fabricated by very low-cost fabrication routes,’ explains Durrant.
‘We don’t use any electricity to split water,’ Domen says. ‘We just put our photocatalyst into the water then add sunlight. The small particles, which are about a few hundred nanometres, evolve hydrogen and oxygen.’ He is currently building a 100m2 pilot plant that he says will be completed this year. The particles currently convert around 1% of the solar energy they receive into chemical energy – a similar efficiency to plants. ‘Our target for the next five to 10 years is 3–5%,’ Domen says. Ultimately he’d like to reach 10%, a goal he describes as ‘not so easy’.
Throwing carbon dioxide into the atmosphere is basically free right now
Domen is working as part of a 10-year, multi-institution artificial photosynthesis collaboration called the Japan Technological Research Association of Artificial Photosynthetic Chemical Process (ARPChem). Industrial collaborators are working to separate the hydrogen and oxygen gas mixture his set-up produces, while other collaborators are using thermocatalysts to use the hydrogen to reduce carbon dioxide into hydrocarbon-based fuels. ‘In this project C2 to C4 hydrocarbons – ethylene, propylene and butene – are our target molecules,’ says Domen.
Large collaborations bringing together academia and industry, such as ARPChem, are increasingly fashionable in this field. As chemists, biochemists and physicists are increasingly ready to reach out to engineers and industry to help them scale up their lab-sized devices into something economically-viable. ‘There’s a huge disconnect at the moment from the fossil-driven economy that operates on the megaton scale versus all these tiny reactors that we have in the labs that operate at a couple of bubbles of hydrogen,’ explains Reisner.
Llobet, for example, is part of E-Scaled, a consortium of academic, private and governmental research organisations and industrial partners from eight European countries. Durrant is part of a large EU consortium – Sunrise – currently bidding for funding through a successor to the current EU flagship programmes.
Coming together to display the power of artificial photosynthesis on the large scale, while hugely important, won’t ultimately guarantee the mainstream success of this technology. Whether or not it has a future depends as much on politics as it does on science. Artificial photosynthesis ‘will never be cheaper than poking a hole in the ground and sucking oil out’, explains Nocera. Carbon pricing is the only way to reverse this. And so far, charging fees for carbon dioxide emissions remains a political hot potato in most parts of the world. ‘Throwing carbon dioxide into the atmosphere is basically free right now,’ adds Llobet. ‘If you had to pay to do that, then these new technologies would probably be much more developed at this point.’ Only time will tell whether we are going to keep aiming for a climate catastrophe, or finally start throwing everything we’ve got at technologies, such as artificial photosynthesis, with the potential to enable us to turn our backs on fossil fuels for good.
Large collaborations bringing together academia and industry, such as ARPChem, are increasingly fashionable in this field. As chemists, biochemists and physicists are increasingly ready to reach out to engineers and industry to help them scale up their lab-sized devices into something economically-viable. ‘There’s a huge disconnect at the moment from the fossil-driven economy that operates on the megaton scale versus all these tiny reactors that we have in the labs that operate at a couple of bubbles of hydrogen,’ explains Reisner. Durrant is part of a large EU consortium – Sunrise – currently bidding for funding through a successor to the current EU flagship programmes.
Coming together to display the power of artificial photosynthesis on the large scale, while hugely important, won’t ultimately guarantee the mainstream success of this technology. Whether or not it has a future depends as much on politics as it does on science. Artificial photosynthesis ‘will never be cheaper than poking a hole in the ground and sucking oil out’, explains Nocera. Carbon pricing is the only way to reverse this. And so far, charging fees for carbon dioxide emissions remains a political hot potato in most parts of the world. Only time will tell whether we are going to keep aiming for a climate catastrophe, or finally start throwing everything we’ve got at technologies, such as artificial photosynthesis, with the potential to enable us to turn our backs on fossil fuels for good.
Nina Notman is a science writer based in Salisbury, UK












1 Reader's comment